-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Frontiers in Science
p-ISSN: 2166-6083 e-ISSN: 2166-6113
2012; 2(6): 144-152
doi:10.5923/j.fs.20120206.03
Antioxidant Activities of Some Spices and Herbs Added to Frozen Chicken Burger
Soumia M. I. Darwish1, Mohamed. A. H. El-Geddawy1, Reda M. B. Khalifa2, Rewaa A. A. Mohamed2
1Food Science and Technology Dept. Faculty of Agric. Assiut Univ. Assiut, 71526, Egypt
2Food Technology Research Institute, Agricultural Research Center, Giza, 12619, Egypt
Correspondence to: Rewaa A. A. Mohamed, Food Technology Research Institute, Agricultural Research Center, Giza, 12619, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The main objective of the present study was carried to evaluate the possibility of using spices and herbs (thyme, rosemary, sage, marjoram and black seeds) as natural antioxidants to help chicken burger industry to minimize lipid oxidation and extend the shelf-life of chicken burger.Two concentrations of these plants (0.5 and 1%) were added to chicken burger and stored at -18℃ for 6 months. Fat content, thiobarbituric acid values (T.B.A) and peroxide value (P.V) were determined. Study indicated that, there were slight increases in crude fat contents of all samples during freezing storage. Furthermore, during storage the thiobarbituric acid values (T.B.A) and peroxide value (P.V) for all samples increased as storage period increased. Meanwhile, treatments which had spices and herbs at levels 1% showed slight decrease in (T.B.A) values and (P.V) compared with control and treatments which had spices and herbs at level 0.5%. Also, the results appeared that thyme was the most efficient protection against lipid oxidation followed by sage, rosemary and then marjoram while black seeds gave less effect. Essential oils of thyme, rosemary, sage, marjoram and black seeds were obtained by steam distillation and the chemical composition of each oils were determined by gas liquid chromatographic technique.The fractionated and identified chemical structures of spices and herbs volatile oilsshowed that , there were 11 components isolated from thyme volatile oil, just 9 components of them were identified. 19 compounds were isolated from rosemary volatile oil, but just 12 components were identified. Chemical composition of sage volatile oil, about 19 compounds were isolated from the oil, only 12 compounds were identified from them. 16 components isolated from marjoram volatile oil, only 14 of them were identified. Finally, 17 components isolated from black seeds volatile oil, only 10 components of them were identified.
Keywords: Antioxidant, Thyme, Sage, Rosemary, Marjoram, Black Seeds
Cite this paper: Soumia M. I. Darwish, Mohamed. A. H. El-Geddawy, Reda M. B. Khalifa, Rewaa A. A. Mohamed, Antioxidant Activities of Some Spices and Herbs Added to Frozen Chicken Burger, Frontiers in Science, Vol. 2 No. 6, 2012, pp. 144-152. doi: 10.5923/j.fs.20120206.03.
Article Outline
1. Introduction
- Changes in eating habits arising from the development of society in recent decades have led people to search for affordable and healthier foods with satisfactory taste and pleasant appearance. Thus, the food industry continually seeks to adapt and develop new formulations designed to increase shelf life and to improve quality and food safety. Chicken meat, especially its industrial products, presents serious problems of processing and storage. Unsaturated lipids, fine grinding, incorporation of air, haem pigments, metal contact and high temperature during processing contribute to lipid oxidation[1]. After microbial deterioration, lipid oxidation is the main process that results in loss of quality[2]. Lipid oxidation generates undesirable products from the sensory point of view, making the food unfit for consumption. In addition, it causes the degradation of fat soluble vitamins and essential fatty acids, and it interferes with the integrity and safety of foods through the formationof potentially toxic compounds[3], such as malonaldehyde (MDA). In an attempt to control this process, food industries use synthetic additives with antioxidant properties. However, due to reports of possible toxic effects from synthetic antioxidants and to increasingly demanding consumerpreferences for natural products and health benefits, the interest foralternative methods to retard lipid oxidation in foods, such as the use of natural antioxidants, has increased. These methods include spice extracts[4], fruit juice[5], tea extracts[6], seed extracts[7] and others. Plants, including herbs and spices, have many phytochemicals which are potential sources of natural antioxidants, e.g. phenolic diterpenes, flavonoids, tannins and phenolic acids[8]. These compounds have antioxidant, anti-inflammatory and anticancer activities[9].The objective of the present study was carried out to investigate the feasibility of using selected natural spices and herbs for improving the lipid stability extending the shelf life of chicken burger during freezing storage and identification of chemical composition of chosen spices and herbs volatile oils.
2. Materias and Methods
2.1. Materials
2.1.1. Chicken Meat
- 10 kg of fresh chicken meat from broiler carcasses (7-8 weeks age with an average weight 1.5-2 kg) were obtained from El-Borssa Company for Poultry at February 2010. On receipt at the laboratory, they were washed carefully then deboned within two hours of slaughtering, the chicken meat was minced using a meat mincer and then chilled at 4±1℃ for 24hours before using in processing of chicken burgers.
2.1.2. Selection of Spices and Herbs
- Selected spices and herbs were used in chicken burger formula namely thyme (thymus vulgaris L.), rosemary (Rosmarinus officinalis L.), black seeds (Nigella sativa L.), sage (Salvia officinalis), and marjoram (Origanum majoranum), were obtain from the Agricultural Research Center, Giza, Egypt.
2.1.3. Salt, Onion, Whole Egg and Bread Crust Powder
- Salt, onion, whole egg and bread crust powder were obtained from the local market and used for preparation of chicken burger. While, soy flour was purchased from the Food Technology Research Institute. Agricultural Research Center-Giza, Egypt.
2.2. Methods
2.2.1. Preparation of Chicken Burger
- Fresh chicken burger samples were prepared as described by[10]. All ingredients were minced twice, after mincing, the chicken mixture was shaped manually using a patty marker (stainless steel model "Form") to obtain round discs 10 cm diameter and 0.5 cm thickness. Burgers were packaged in polyethylene bags (in foam dishes).• The Basal constituents of chicken burger were prepared as follows:The chilled minced chicken meat formula included fat 71.5%, fresh onion (finely ground) 7.0%, whole egg (blended) 5.0%, bread crust powder 5.0%, rehydrated extruded soy 10.0% and sodium chloride 1.50%. These ingredients were mixed together, divided to eleven equal portions, the first portion was remained without any addition (control) and the ten reminder portions were individually mixed with two concentrations of each spices and herbs (0.5% and 1%) to give ten treatments . All burgers treatments and control were freeze stored at -18±2ºC up to 6 months.
2.2.2. Analytical Methods
- Fresh chicken meat used in this study was analyzed immediately upon receipt at the laboratory for chemical analyses, as well as immediately after manufacturing (zero- time analyses), and then after 1, 2, 3, 4, 5 and 6 months of frozen storage at -18±2℃.
2.2.2.1. Crude Fat Contents
- Crude fat determined according to the methods described by[11].
2.2.2.2. Thiobarbituric Acid Value (T.B.A)
- Thiobarbituric acid (TBA) values were determined in chicken burger samples at 0, 1, 2, 3, 4, 5 and 6 months of storage at -18℃ according to the method of[12] to evaluate efficiency of additives as natural antioxidants. Twenty g sample plus 40 ml of trichloroacetic acid (7.5%) were homogenized for 1 minute and left for 30 minutes. Filtration was carried out using whatman No. 1 filter paper. Five ml of the filtrate mixed with 5 ml of TBA solution (0.2883 g TBA/ 100 ml water) in a test tube. Blank was carried out using 5 ml distilled water and 5 ml TBA solution. Tubes were covered and heated in boiling water bath for 40 min, then after rapid cooling in ice bath, absorbance at 538 nm was measured using ultraviolet visible scanner Spectrophotometer (LKB 4054 Cambridge, England). The TBA values were calculated by multiplying the absorbance by the factor of 7.8 and the result was represented as mg of malonaldehyde per 1000g sample.
2.2.2.3. Peroxide Value (P.V)
- Weight 5.00±0.05 g sample into 250 mL glass-stoppered Erlenmeyer. Add 30 mL CH3 COOH- CHCl3 and swirl to dissolve. Add 0.5 mL saturated Kl solution from Mohr pipet, let stand with occasional shaking 1 min, and add 30mL H2O. Slowly titrate with 0.1 N NA2S2O3 with vigorous shaking until yellow is almost gone. Add ca 0.5 mL 1% starch solution, and continue titration, shaking vigorously to release all I2from CHCl3 layer, until blue just disappears. If <0.5 mL 0.1N Na2So3 is used Conduct blank determination daily (must be <0.1 mLO 0.1N Na2 So3). Subtract from sample titration.Peroxide value (milliequivalent peroxide/ Kg sample) = S x N x 1000/g sample, where S= mL Na2So3 (blank corrected) and N normality Na2S2O3 solution[11].
2.2.2.4. Extraction of Essential Oils
- The volatile oils of chosen spices and herbs (used in burger formula) were extracted from their spice and herbs, using steam distillation method in a glass apparatus for 4-6 hours. The extracted volatile oils were dried over anhydrous sodium sulphate before held in dark glass bottles at - 20℃ according to the method of[11].
2.2.2.5. Classification and Identification of Essential Oil Components in Spices and Herbs
- • Gas chromatographic analyses:The volatile oil was analyzed using DsChrom 6200 Gas Chromatographic equipped with a flame ionization detector for separation of volatile oil constituents in Medicinal and Aromatic Plants, Horticulture Research Institute, Agricultural Research Center according to method described by[13]. The analyses conditions were as follows:1-The chromatographic apparatus was fitted withcapillary column BPX-5, 5% phenyl (equiv.) polysillphenylene- siloxane 30m x 0.25mm ID x 0.25μm film.2-Temperature program ramp increase with a rate of 10℃/min from 70º to 200℃.3-Flow rates of gases were nitrogen at 1 ml/min, hydrogen at 30ml/min and 330ml/min for air. Detector and injector temperatures were 300℃ and 250℃, respectively. The obtained chromatogram and report of GC analyses for each sample were analyzed to calculate the percentage of main components of volatile oil.
2.2.2.6. Statistical Analyses
- The data obtained from three replicats were analyzed by ANOVA using the SPSS statistical package program, and differences among the means were compared using the Duncan’s Multiple Range test[14]. At a significance level of 0.05 was chosen.
3. Results and Discussion
3.1. Crude Fat Contents
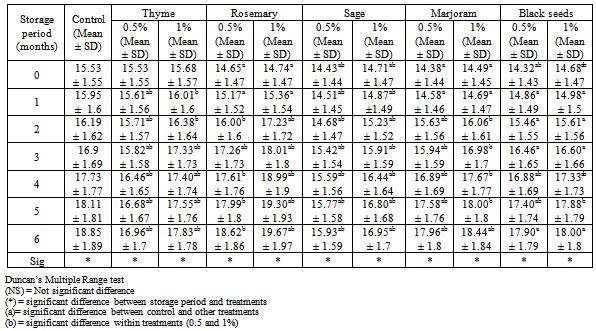
- Data in Table (1) showed that, crude fat contents for all chicken burger samples during freezing storage at -18℃ up to 6 months. The results cleared that, during frozen storage there were slight increase with a significant difference in crude fat contents of all different chicken burger treatments under investigation. These increments in crude fat contents of chicken burger during frozen storage might be due to the decrease of the moisture and crude protein contents during storage. These results are in line with the findings of[13,15, 16]. Furthermore, data in the same table revealed that there were a significant difference between control samples and other treatments. Also, there were significant differences in comparison within treatments (0.5 and 1.0%) in most cases.
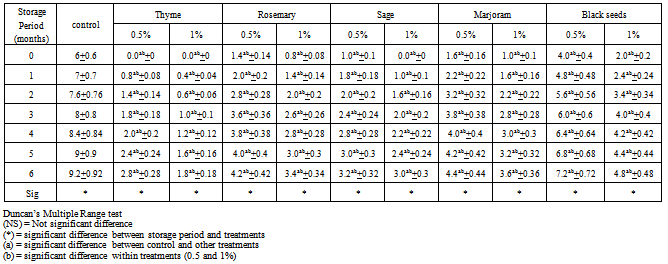
3.2. Thiobarbituric Acid (T.B.A) Values
- Thiobarbituric acid (T.B.A) test is used as an index for measuring oxidative rancidity (malonaldehyde formation) which takes place in chicken meat. The T.B.A test is a sensitive test for the decomposition products of highly unsaturated fatty acids which do not appear in peroxide value determination[17].Results in Table (2) indicated that the changes of thiobarbituric acid T.B.A values during frozen storage at -18℃ up to 6 months for spiced (with powdered spices and herbs) or unspiced chicken burger (as a control), determined as a mg malonaldehyde / kg sample. Data showed that, during frozen storage, the T.B.A value of the control sample showed continuous progressive increases to reach the highest value (0.700 mg malonaldehyde/kg) at the end of frozen storage periods. Although the other burger treatments showed increment in T.B.A values during frozen storage, these increments were largely lower than that of control sample with a significant difference in some cases. In regard to these results, burger samples formulated with spices and herbs at level 1% had the lowest T.B.A values at the end of frozen storage, while those samples formulated with spices and herbs at level 0.5% had higher T.B.A values with no significant difference compared within each treatment. The slight increases in T.B.A values of different chicken burger treatments compared with control, could be attributed to the antioxidant activity of some spices and herbs components especially their volatile oils. This concept is on line with[18]. Generally, during storage the T.B.A values of all samples increased with no significant difference as storage periods increased. These results were in line with[19,20].
|
|
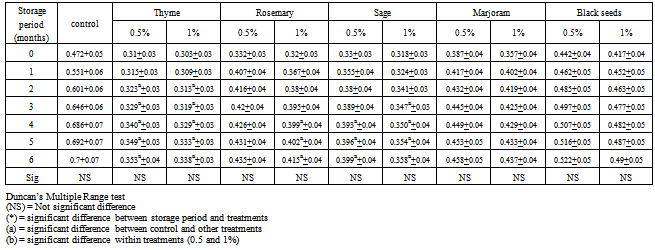
3.3. Peroxide Values (P.V)
- The peroxide values are used as an index of the degree of oxidative rancidity of lipids. According to[26], fresh edible animal fats which might be stored for long periods, show peroxide values of 2.34 m. equiv./kg fat or less. In comparison with vegetable oils, this value was lower than the maximum permissible amount given for sun flower seed oil (not more than 10. m. equiv. / kg fat).Table (3) cleared that the changes peroxide values of spiced chicken burgers and control sample during frozen storage at -18℃ up to 6 months. The recorded data revealed that peroxide values (P.V) had the same trend of T.B.A values. Also, peroxide values in all chicken burger treatments tended to significant increase with the progressive of frozen storage period. These findings are in the line with those obtained by[27,28] and[13]. During freezing storage the chicken burger samples formulated with spices and herbs at level 1% had lower P.V values than those samples formulated with spices and herbs at level 0.5% with a significant difference in all cases when compared within treatments. Furthermore, the control sample had higher P.V value compared with other treatments, this could be attributed to the antioxidant activity of some spices and herbs components. Also, during frozen storage the sample formulated with thyme at level 1% caused more reduction than the P.V compared to sample formulated with thyme at level 0.5% and other treatments. At the same time P.V values recorded in samples formulated with sage at levels 0.5 and 1% were lower than those samples formulated with rosemary at levels 0.5 and 1%, respectively. Meanwhile, the P.V values in samples formulated with rosemary at levels 0.5 and 1% were lower than those samples formulated with marjoram at levels 0.5 and 1%. Furthermore, the P.V values in samples formulated with marjoram at levels 0.5 and 1% were lower than these samples formulated with black seeds at levels 0.5 and 1%.However, it could be summarized that the addition of spices and herbs at level 1% caused decrement in P.V values in fresh chicken burger compared with chicken burger formulated with spices and herbs at level 0.5% and unspiced one (control). The present of thyme in chicken burger showed the highest for reducing P.V value in samples. The effectiveness of decrement followed the sequence: Thyme > sage > rosemary > marjoram > black seeds. Similar results were obtained by[23].
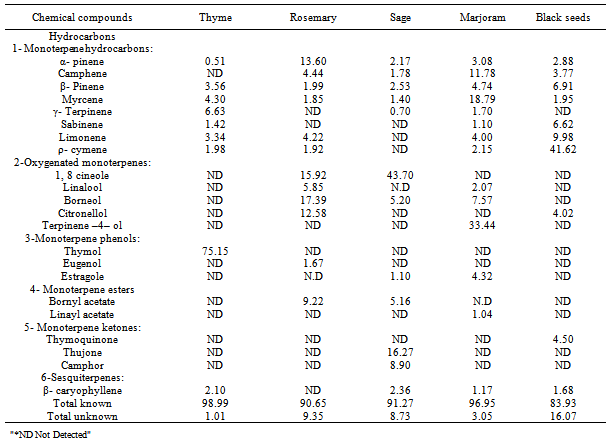
3.4. Classification and Identification of Spices and Herbs Volatile Oils
- The essential oil was extracted from the investigated spices and herbs by the steam distillation method. In an attempt to define the identity and roles played by chemical components of spices and herbs volatile oils as antioxidant and antimicrobial agents, gas chromatography (GC) was used to fractionate and identify these compounds. The fractionated and identified chemical structures of spices and herbs volatile oils were tabulated in Table (4).From the data tabulated in Table (4) it could be observed that, there were 11 components isolated from thyme volatile oil, just 9 components of them were identified. The identified compounds represented 98.99% of the chemical components of thyme volatile oil and could be classified to three chemical categories. The identified chemical groups namely; monoterpene hydrocarbon (21.74%), monoterpene phenols (75.17%) and sesquiterpenes (2.10%). On the other hand, the remaining portion of this volatile oil was (1.01%) represented 2 unknown compounds, might be considered as trace compounds in such oil.Results in Table (4) showed that the first chemical group identified in thyme oil was monoterpene hydrocarbons which contained 7 components namely: α-pinene (0.51%), sabinene (1.42%), β-pinene (3.56%), myrcene (4.30%), limonene (3.34%), ρ-cymene (1.98%) and γ-terpinene (6.63%). The second group was monoterpene phenols which included thymol (75.15%) which was the major component. The third chemical group was sesquiterpenes which contain β-cayophllene (2.10%).[29] determined the major components of thyme essential oil by GC and GC-Mass and they were (carvacrol, thymol, p-cymene and 1,8 cineole).[30] identified the aroma compounds in the extracts of thyme leaves by (GC) and (GC/MS). The major aroma constituents of thyme were thymol (8.55mg/g), carvacrol (0.681 mg/g), linalool (0.471 mg/g), α-terpineol (0.291 mg/g) and 1,8 cineole (0.245mg/g). From the Table (4) it could be noticed that, 19 compounds were isolated from rosemary volatile oil, but just 12 components were identified. Those obtained compounds represented (90.65%) of chemical components of rosemary volatile oil, these compounds could be classified to four chemical categories, namely; monterpene hydrocarbons (28.02%), oxygenated monoterpenes (51.75%), monoterpene esters (9.22%) and monoterpene phenols (1.67%). The remaining portion of volatile oil (9.35%), represented 7 unknown compounds might be considered as trace compounds in such oil.Results in the same table showed that the first chemical group identified in the volatile oil of rosemary was monoterpene hydrocarbons (28.02%) which consist of 6 components namely; α-pinene (13.60%), camphene (4.44%), β-pinene (1.99%), myrcene (1.85%), limonene (4.22%) and ρ-cymene (1.92%). The second identified chemical group was oxygenated monterpenes (51.75%) which consisted of four components were found namely; 1,8-cineole (15.92%), linalool (5.85%), borneol (17.39%) and citronellol (12.58%). The third identified group was monoterpene esters which contained only one compound was bornyl acetate (9.22%). The fourth chemical group identified in rosemary volatile oil was monoterpene phenols which contained only one compound namely; estragole (1.67%). In this concern[31] found that the analysis of the essential oil of rosemary by gas chromatography- mass spectrometry showed that eucalyptol, vorneol, α-pinene, α- terpineol, camphor and camphene constituted 45.80%, 16.51%, 6.04%, 5.55%, 3.00% and 2.22% of this essential oil. While,[32] found that, the essential oil for rosemary contained α-pinene 23.06%, camphene 4.50%, β-pinene 2.16%, limonene 3.33%, 1,8-cineole 8.91%, γ-terpinene 1.92%, terpinolene 1.36%, linalool 1.95%, camphor 3.12%, borneol 3.49%, terpinen-4-ol 1.52%, α-terpineol 1.70%, myrtenol 1.32%, verbenone 24.11%, geraniol 1.79%, bornyl acetate 5.50%. All these compounds represented 89.74% of essential oil compounds.The same table showed the chemical composition of sage volatile oil, about 19 compounds were isolated from the oil, only 12 compounds were identified from them. The identified compounds represented (91.27%) from the whole oil constituents and were classified for 6 chemical groups, namely; monoterpene hydrocarbons (8.58%), oxygenated monoterpenes (48.90%), monoterpene ketones (25.17%), monoterpene esters (5.16%), monoterpene phenols (1.10%) and sesquiterpenes (2.36%). The first identified chemical group in Table (4), sage volatile oil was monoterpene hydrocarbons, which consisted of five compound namely; α-pinene (2.17%), camphene (1.78%), β-pinene (2.53%), myrcene (1.40%) and γ-terpinene (0.70%). The second chemical groups was oxygenated monoterpenes which contained 1,8 cineole (43.70%) and borneol (5.20%). The third group (25.17%) was monoterpene ketones which contained thujone (16.27%) and camphor (8.90%). The fourth chemical group was monoterpene esters, which contained bornyl acetate (5.16%). The fifth chemical group of sage volatile oil was monterpene phenols, which contained only one compound eugenol (1.10%). The sixth chemical group fractionated and identified in sage volatile oil was sesquiterpenes which contained one compound namely; β- caryophllen (2.36%). Furthermore, the remaining portion of this volatile oil (8.73%) represented 7 unknown compounds might be considered as trace compounds.The chemical composition of sage essential oil has been investigated in various countries.[33] found that the essential oil from sage originating from Jordan contains α-pinene, camphene, limonene, 1,8-cineole, α and β-thujone, camphor, lonalool, linalyl acetate, bornyl acetate and humulene. In the essential oil 29 components were detected, 28 of them were identified and a dominant share had α-thujone (29.9%), β-thujone (13.68%), camphor (15.74%) and 1,8-cineole (12.31%).[34] found that GC-MS analyses of sage essential oils identified 37 constituents, representing 90.0% of the total oil. The main components were camphor (24.95%), 1,8-cineole (24.75%) and camphene (7.63%).From the results illustrated in Table (4), it could be indicated that there were 16 components isolated from marjoram volatile oil, only 14 of them were identified. These components could be classified into five chemical categories namely; monoterpene hydrocarbons (47.34%), oxygenated monterpenes (43.08%), monoterpenes esters (1.04%), monoterpenes phenols (4.32%) and esequiterpenes (1.17%). These identified compounds accounted for (96.95%) of the composition of marjoram volatile oil. The remaining portion (3.05%) was represented by 2 unknown constituents.
|
|
4. Conclusions
- Results of the present study demonstrate the positive effects of spices and herbs, added with two concentrations on both retarding lipid oxidation and improving shelf life of chicken burger during frozen storage (-18℃) for 180 days. The best results were obtained with the concentration of spices and herbs at level 1% to improve preservation of chicken burger. The highest antioxidant effect was recoded for thyme, followed by sage, rosemary and then marjoram while black seeds gave less inhibitory effect. In addition to, the gas chromatographic (GC) analysis of thyme, rosemary, sage, marjoram and black seeds volatile oils, indicated that the number of identified components in these volatile oil were 9, 12, 12, 14 and 10 compounds, respectively. These compounds representing 98.99, 90.66, 91.27, 96.95 and 83.93% of the structure of these five volatile oils, respectively. The major compound of these volatile oils were thymol (75.15%) for thyme oil, 1,8 cineole (15.92%) and borneol (17.39%) for rosemary oil, 1,8 cineole (43.70%) and thujone (16.27%) for sage oil, terpinene-4-ol (33.44%) for marjoram oil and ρ-cymene (41.62%) and thymoquinone (4.50%) for black seeds oil. Spices, herbs and their essential oils (EOs) are used by the food industry as natural agents for extending the shelf life of foods. A variety of plant- and spice-based antioxidants and antimicrobials is used for reduce oxidation, eliminating pathogenic bacteria, and increasing the overall quality of food products.
ACKNOWLEDGEMENTS
- The authors thanks Meat and Fish Technology Department Research Institute and Medicinal and Aromatic Plants, Horticulture Research Institute, Agriculture Research center (A.R.C), Ministry of Agriculture, Giza, for support and helps to obtain spices and herbs which used in this investigation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML