-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Frontiers in Science
p-ISSN: 2166-6083 e-ISSN: 2166-6113
2012; 2(5): 127-132
doi: 10.5923/j.fs.20120205.05
Nephrotoxic Effect of Sub-Acute Exposure of Treated Carbanaceous Effluent on Mice
Agunbiade SO 1, Daramola OT 1, Anugweje KC 2, Okonko IO 3
1Department of Biochemistry, Lead City University, Ibadan, Nigeria
2Department of Health Services, Lulu Briggs Health Centre, University of Port Harcourt, East-West Road, P.M.B. 5323, Choba, Port Harcourt, Rivers State, Nigeria
3Department of Microbiology, University of Port Harcourt, East-West Road, P.M.B. 5323, Choba, Port Harcourt, Rivers State, Nigeria
Correspondence to: Okonko IO , Department of Microbiology, University of Port Harcourt, East-West Road, P.M.B. 5323, Choba, Port Harcourt, Rivers State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Neurotoxicity tests in animals exposed to toxic substances could provide additional information on possible neurotoxic effects. The aim of this study is to determine the nephrotoxic effect of wastewater discharged from a carbonaceous industry on mice (Mus musculus). The present study examined the nephrotoxic effects of carbonaceous wastewater in mice, the mice were exposed to five different concentrations of the waste water. Cyclophosphomide was used as the positive control and distilled water was used as a negative control, for a period of 35das. Creatinine and urea concentration in serum was used as kidney function. The weights of the animals were recorded weekly after which their kidneys were harvested. Organ weight was measured at post exposure and preserved afterwards for histology. The physical, chemical and heavy metal composition of the wastewater was also analysed. There was no significant (P>0.05) change in the kidney and body weight of the exposed mice or the negative control. The activities of urea and creatinine in the serum of exposed mice were significantly increased compared to the negative control mice and this increase was concentration dependent at P<0.05. The histological lesions observed in the kidney showed generalised occlusion of the tubular lumen, general tubular necrosis, with protein casts in the tubular lumen and multiple foci of haemorrhage in the parenchyma were observed. The results of the study showed that the observed nephrotoxic effect in the exposed mice may be caused by the presence of heavy metal and other physical and chemical substances present in the waste water. This suggests a higher risk to kidney damage in humans and other organisms exposed to this waste water and may also be deleterious to the surrounding environment.
Keywords: Creatinine, Urea, Carbonaceous Effluent, Nephrotoxic Effect, Mice, Wastewater
Cite this paper: Agunbiade SO , Daramola OT , Anugweje KC , Okonko IO , "Nephrotoxic Effect of Sub-Acute Exposure of Treated Carbanaceous Effluent on Mice", Frontiers in Science, Vol. 2 No. 5, 2012, pp. 127-132. doi: 10.5923/j.fs.20120205.05.
Article Outline
1. Introduction
- Toxicology can be defined as a branch of science that deals with poisons. A poison is any substance that causes a harmful effect when administered, either by accident or design, to a living organism. The word ‘poison’ is a quantitative concept as almost any substance can be harmful at some doses but not harmful at others[1].Neurotoxicity tests in animals exposed to toxic substances could provide additional information on possible neurotoxic effects[2]. Humans are continuously exposed to different chemicals from water, air and soil everyday. The kidneys are important organs for metabolism, detoxification, storage and excretion of these chemicals and their metabolites and thus are vulnerable to damage. The similarity in body weight of mice exposed to wastewater and control may be due to the ability of the mice to feed even while being exposed. The increase in kidney may be due to inflammation of these chemicals present in waste water. Such observations were also observed in rats exposed to cadmium and alcohol[3]. Indiscriminate discharge of untreated or partially treated wastewaters directly or indirectly into aquatic bodies may render water resources unwholesome and hazardous to man and other living systems[4-9].Several reports have demonstrated acute toxicity of industrial effluents to microalgae, fishes and bacteria. However reports on the nephrotoxicity are few and are mostly with microbial assays. More often than not, landfills of these effluents neither have a synthetic membrane liner at the bottom, nor a natural layer of compacted soil with the desired hydraulic conductivity, nor a run-off control system[9]. This is a potential source of leechates which ultimately find their way into municipal and local water supplies, adding to the toxic components of domestic water we use every day[9].An imperative need has been suggested to examine the toxic and nephrotoxic effects of these wastewaters in discharged from a carbonaceous industry on mice in order to generate information that will be useful to the environmental authorities for promulgating laws that will guide the proper management of these waste waters[4-6, 9-11].Considering the high correlation between mutagenicity and carcinogenicity, it may be pertinent to mention that humans predisposed to cancer and exposed sewage systems which are heavy laden with improperly treated waste effluents may be at a very high risk for developing the disease[9]. This is because of the chemical content of industrial effluents that are active in initiation, promotion and progression and may work in concert to bring about neoplastic transformation[9, 12-14]. Thus, there are substances in the test sample that are capable of inducing genetic effects in mice which is relevant to human health because the toxicological target is DNA, which exists in all cellular forms[9,15]. The aim of this study is to determine the nephrotoxic effect of wastewater discharged from a carbonaceous industry on mice (Mus musculus). The aim would be achieved through examination of Kidney function test. These will include the monitoring of urea and creatinine[16], histopathology of the kidney, body and organ weight index. The waste water will also be analysed for the presence of some physical, chemical and heavy metals. The findings from this study may be useful in the assessment of the toxic effects of waste water samples on public health and environment. This may be a useful public marker in the promulgation of stringent decisions as regards to the indiscriminate disposal of water.
2. Materials and Methods
2.1. Sources of Experimental Materials and Treatment
2.1.1. Laboratory Mice
- Forty two male albino mice were obtained from the animal breeding unit at the Institute for Advanced Medical Research and Training (IMRAT), College of Medicine, University of Ibadan, UCH, Ibadan, Nigeria. Mice were acquired and quarantined in a pathogen-free, well ventilated room in order to enable the animals acclimatize to their environment. During the period of acclimatization, the animals were supplied with food (pelleted foods) and drinking water on a daily basis. Their beddings were also changed daily (disinfected and discarded). The mice were maintained in the departmental animal and breeding unit at IMRAT where each cage contains six animals.
2.1.2. Waste Water
- The waste water sample was sourced from the drainage pipes of the Effluent Treatment Plant of a beverage producing company in Lagos State, Nigeria. This company is well known for the production of drink beverages which are consumed nationwide.
2.1.3. Storage of Effluent
- The collected effluents were stored in plastic bottles and refrigerated at 4°C until when needed. They were then brought out and diluted to various concentrations at room temperatures. The various concentrations were in-turn stored in plastic bottles and refrigerated all through the experiment.
2.2. Preparation of Controls
2.2.1. Negative Control
- Distilled water was used as a vehicular solvent for the dilution of the waste water used.
2.2.2. Positive Control
- The drug cyclophosphamide was used. Administered dosage depends on the average body mass of the animal per kg, which is 40mg/kg. The value/information in mg provided by the manufacturer was taken into consideration when the calculation was made. Average body weight of animal for positive control per kg is 24.5g; 40mg ---à 1000g; X ---à 24.5g; X = 40 x 24.5/1000 = 0.98mg of cyclophosphamide dissolved in 1000ml distilled water and administered orally for 35 days.
2.3. Exposure of Animals to the Samples
- The animals were randomly divided into seven groups. Each group was made up of six individuals. Five groups were each injected with a different dilution of the effluent. One group was administered a positive control and another group the negative control sample. The dosage that was administered depends on the average body weight. 0.3ml of the sample was administered orally to each animal for a period of 35 consecutive days.
2.4. Serum Creatinine Estimation
- This was carried out as described by Cheesbrough[16]. Creatinine in alkaline solution reacts with picric acid to form a coloured complex. The amount of complex formed is directly proportional to the creatinine concentration.
2.5. Serum Urea Estimation
- This was carried out as described by Cheesbrough[16]. Urea in the serum is hydrolysed to ammonia in the presence of urease. The ammonia is then measured photometrically by Berthelot’s reaction.
2.6. Tests and Methodology
- pH determination, alkalinity, total sulphate, total chloride, total hardness, total dissolved solids, total suspended solids, biochemical oxygen demand (BOD), chemical oxygen demand (COD) and determination of metals.
2.7. Statistical Analysis
- Results were analysed by the mean standard deviation and statistical analysis of variance (ANOVA).
3. Results Analysis
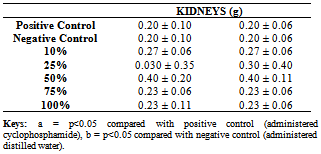
- Table 1 shows the physico-chemical parameters of the industrial carbonaceous waste water used in this study.
|
|
|
4. Discussion
- The present study examined the nephrotoxic effects of carbonaceous wastewater in mice, the mice were exposed to five different concentrations of the waste water. The present study showed that heavy metals concentrations were on the high side. This finding is therefore useful in further assessment of the toxic effects of chemicals present in the wastewater samples on the environment if dumped indiscriminately. Compared to the allowable limits[9, 17-19] most of the parameters analyzed, especially the heavy metals were present in high concentrations. This is comparable to the findings of previous studies[6, 9, 20-23]. Ubalua[20] stated that the claim that cassava wastewater can cause problems in some crops is based on anecdotal information.Also in the present study, there was a concentration-dependent increase in serum urea and creatinine when compared to the control at the p<0.05 level. The kidney damage probably contributed to their increase. Orisakwe et al.[24] reported similar conditions in rats exposed to Hibiscus Sabdariffa Calyx.The histological lesions observed in the kidney showed generalised occlusion of the tubular lumen, general tubular necrosis, with protein casts in the tubular lumen and multiple foci of haemorrhage in the parenchyma were observed. The results of the study showed that the observed nephrotoxic effect in the exposed mice may be caused by the presence of heavy metal and other physical and chemical substances present in the waste water. This is in line with similar observation made in other related studies by some authors. Freund[25] observed small hemorrhages in the bronchi and trachea of a person who died from accidental exposure to vapors of Nitrosodimethylamine (NDMA). Gastrointestinal hemorrhage was observed in a person who died from accidental exposure to vapors of NDMA[2,25]. Jenkins et al.[26] also observed mortality in rats that received NDMA for 9 weeks. According to ATSDR/USEPA[2] reports, mice that received NDMA in drinking water for 224 days did not experience significantly decreased survival[27].In a study by Maduagwu and Bassir[28], daily gavage exposure to NDMA for 30 days caused decreased survival in cats. According to Ungar[29] and ATSDR/USEPA[2] reports, daily administration of the same substance in drinking water for 8, 12, or 16 weeks resulted in occasional moribundity in hamsters, while no lethality resulted from daily administration of the same substance for 28 days[2, 30]. In a study by Nishie[31], pregnant and nonpregnant rats were treated with a single NDMA dose. An unspecified number of deceased animals had distal tubule necrosis two days following treatment, and surviving rats had normal kidneys. Macroscopic congestion was noted in kidneys of rats that were administered with NDMA in the diet for l-12 weeks[2,32]. Moderate tubule congestion with other effects (glomerulus dilatation, slightly thickened Bowman's capsule) were observed in mink that ingested the same substance via diet[2,33]. In a related study, Olorunfemi et al.[23] reported that heavy metals-cyanide interaction in the cassava waste waters was responsible for the anomalies in cell division process and chromosome aberration induction in the Allium cepa root meristem. Also in another related study conducted by Adeyemo[34] to assess the haematological and histopathological effects of cassava effluent on adult female African catfish, Clarias gariepinus, the fish was found to show signs of gill and liver damage. Similarly, histopathological examination of the kidney, gill and liver of the fingerlings of the Nile Tilapia, Oreochromis niloticus treated with cassava effluent indicated damage[35].Ivanova et al.[36] and Staykova et al.[37] have established the genotoxic and mutagenic effects of open water contaminated with heavy metals and cyanide, further confirming the results of the inhibitory effects of these effluents in previous studies[9, 21-23]. In another study by Agunbiade et al.[9], the genotoxic and histopathological effects of the effluents from carbonaceous bottling plant were established. The study indicates that the effluents contain toxic substances which may constitute a risk to the environment and human health, more especially as the waste generated from effluents from carbonaceous bottling processing plant is not properly managed[9]. In mammals, several sub-chronic exposures of rats, mice and monkeys to substances such as perfluorooctanesulfonate (PFOS) have resulted in effects on body weight gain in females and in males[38-44] as reported in this present study with carbonaceous effluent. The results of the study by Agunbiade et al.[44] showed that the observed hepatotoxic effect in the exposed mice may be caused by the presence of heavy metal and other physical and chemical substances present in the wastewater. It suggested a higher risk to liver damage in humans and other organisms exposed to this wastewater and may also be deleterious to the surrounding environment[44].However, limited information is available regarding renal effects of orally administered wastewater discharged from a carbonaceous industry in animals.
5. Conclusions
- In this study, there was no significant (P>0.05) change in the kidney and body weight of the exposed mice or the negative control. However, the activities of urea and creatinine in the serum of exposed mice were significantly increased as compared to the negative control mice. Our study showed that this increase in the activities of urea and creatinine in the serum of exposed mice was concentration dependent (P<0.05). The findings of this study also showed that the histological lesions observed in the kidney were characterized by generalized occlusion of the tubular lumen, general tubular necrosis, with protein casts in the tubular lumen and multiple foci of haemorrhage in the parenchyma. The carbonaceous wastewater caused kidney dysfunction in mice at various concentrations. This suggests that exposure to these waste may pose risk to human health and will pollute the aquatic environment, contaminating the source of water supply for both domestic and commercial uses. Our results showed that the observed nephrotoxic effect in the exposed mice may be caused by the presence of heavy metal and other physical and chemical substances present in the wastewater. This suggests a higher risk to kidney damage in humans and other organisms exposed to this wastewater and may also be deleterious to the surrounding environment.
ACKNOWLEDGMENTS
- The authors expresses their sincere appreciation to the management and staff of the animal breeding unit, Institute for Advanced Medical Research and Training (IMRAT), College of Medicine, University of Ibadan, UCH, Ibadan, Nigeria for the forty-two male albino mice used for this study.
References
| [1] | Hodgson, E. And Smart, R. C. (Eds), 2001. Introduction to Biochemical Toxicology. 3rd Edition. New York. |
| [2] | ATSDR/USEPA. 1989. Toxicological Profile For N-Nitrosodimethylamine. Agency for Toxic Substances and Disease Registry (ATSDR) U.S. Public Health Service In collaboration with U.S. Environmental Protection Agency (EPA). U.S. Government Printing Office (1991-535-152), pp1-119, |
| [3] | Brzooska, M. M., Moniuszka-Jakomiuk, J., Kiewicz, P. B. 2003. Liver and Kidney Function and Histology in Mice Exposed to Cadmium and Ethanol. Alcohol and Alcoholism. 38(1):2-10 |
| [4] | Bakare AA, Mosuro AA, Osibanjo O. Landfill Leechate Induced Toxicity in Mice. J. Environ. Biol., 2003a; 24(4), 429-435. |
| [5] | Bakare AA, Lateef A, Amuda OS, Afolabi RO. The aquatic toxicity and characterization of chemical and microbiological constituents of water samples from Oba River, Odo-Oba, Nigeria. Asian J. Microbiol. Biotechnol. Environ. Sci., 2003b, 5: 11-17. |
| [6] | Bakare AA, Okunola AA, Adetunji OA, Jenmi HB. Genotoxicity assessment of a pharmaceutical effluent using four bioassays. Genet. Mol. Biol., 2009; 32: 373-381. |
| [7] | Fawole, O.O., T.A. Yekeen, A.A. Ayandele, A. Akinboro, M.A. Azeez and S.O. Adewoye, 2008. Polluted Alamuyo River: Impacts on surrounding wells, microbial attributes and toxic effects on Allium cepa root cells. Asian J. Biotechnol., 7: 450-458. |
| [8] | Kumar ARG. Anaphase-telophase aberration assay of fertilizer factory effluent in Allium cepa L. J. Cytol. Genet., 2008; 9: 131-135. |
| [9] | Agunbiade SO, Okonko IO, Alimba CG, Folarin AC, Anugweje KC. 2012. Effects Of A Carbonaceous Bottling Plant Effluent On Albino Mice Sperm Morphology and Testes Histopathology. Nature and Science; 10(8):154-160]. |
| [10] | Babatunde, B.B. and A.A. Bakare, 2006. Genotoxicity screening of wastewaters from Agbara industrial estate, Nigeria evaluated with the Allium test. Pollut. Res., 25: 227-234. |
| [11] | Bakare AA, Wale-Adeyemo AR. The mutagenic and cytotoxic effects of leacheates from domestic solid wastes and Aba-Eku landfill, Nigeria on Allium cepa. Nat. Environ. Pollut. Technol., 2004; 3: 455-462. |
| [12] | Fowler BA, Maehle L, Mollerup S, Rivedal E, Ryberg D. Role of lead binding proteins in renal cancer. Environmental Health Perceptive, 1994; 102: 115-116 |
| [13] | Haugen A, Maehle L, Mollerup S, Rivedal E, Ryberg D. Nickel-induced alterations in human renal epithelial cells. Environmental Health Perceptive, 1994; 102: 117-118 |
| [14] | Elinder CG, Jarup L. Cadmium exposure and health risks: recent findings. Ambio, 1996; 25(5): 370-373 |
| [15] | Houk VS. The genotoxicity of industrial wastes and effluents: A review. Mut. Res., 1992; 277:91-138 |
| [16] | Cheesebrough M. 2006. District Laboratory Practice in Tropical Countries, part 1. University Press, Cambridge, pp. 239-258. |
| [17] | Federal Environmental Protection Agency (FEPA). 1991. S1.8 National Environmental Protection (Effluent Limitations) Regulations 1991 as Cited by Odiete. In: Environmental Physiology of Animals and Pollution, Okoye, B.C.O. (Ed.). Diversified Resources Ltd., Lagos, Nigeria, pp: 157-219. |
| [18] | United States Environmental Protection Agency (USEPA). EPA report to congress: Solid waste disposal in the United States. EPA Office of Solid Waste and Emergency Response, Volume 1. EPA/530-SW-8-011, Washington D.C., 1988. |
| [19] | UNESCO/WHO/UNEP. The Selection of Water Quality Variables. In: Water Quality Assessments, A Guide to the Use of Biota, Sediments and Water in Environmental Monitoring, Chapman, D.V. (Ed.). 2nd Edn., Chapman and Hall Ltd., London, 1992; pp: 51-119. |
| [20] | Ubalua AO. Cassava wastes: Treatment options and value addition alternatives. Afr. J. Biotechnol., 2007; 6: 2065-2073. |
| [21] | Olorunfemi D, Obiaigwe H, Okieimen E. Effect of cassava processing effluent on the germination of some cereals. Res. J. Environ. Sci., 2007; 1: 166-172. |
| [22] | Olorunfemi DI, Emoefe EO, Okieimen FE. Effect of cassava processing effluent on seedling height, biomass and chlorophyll content of some cereals. Res. J. Environ. Sci., 2008; 2: 221-227. |
| [23] | Olorunfemi DI, Okoloko GE, Bakare AA, Akinboro A. Cytotoxic and Genotoxic Effects of Cassava Effluents using the Allium cepa Assay. Research Journal of Mutagenesis, 2011; 1: 1-9. |
| [24] | Orisakme, O. E., Hussaini, O. C., Orish, V. E. N., Udemezue, O. O.2003. Nephrotoxic Effect of Hibiscus Sabdariffa Calyx in Rats. European Bulletin of Drug Research. 1194: 99-103. |
| [25] | Freund HA. 1937. Clinical manifestations and studies in parenchymatous hepatitis. Ann Int Med 10:1144-1155. |
| [26] | Jenkins SA, Grandison A, Baxter JN, et al. 1985. A dimethylnitrosamineinduced model of cirrhosis and portal hypertension in the rat. J Hepatol 1:489-499. |
| [27] | Clapp NK, Toya RE Sr. 1970. Effect of cumulative dose and dose rate on dimethylnitrosamine oncogenesis in RF mice. Journal of National Cancer Inst 45:495-498. |
| [28] | Maduagwu EN, Bassir O. 1980. A comparative assessment of toxic effects of dimethylnitrosamine in six different species. Toxicol Appl Pharmacol 53:211-219. |
| [29] | Ungar H. 1986. Venoocclusive disease of the liver and phlebectatic peliosis in the golden hamster exposed to dimethylnitrosamine. Path Res Pratt 181:180-187. |
| [30] | Ungar H. 1984. Primary portal venopathy in the golden hamster treated with low doses of dimethylnitrosamine. Liver 4:244-254. |
| [31] | Nishie K. 1983. Comparison of the effects of N-nitrosodimethylamine on pregnant and nonpregnant Holtzman rats. Fd Chem Toxicol 21:453-462. |
| [32] | Khanna SD, Puri D. 1966. The hepatotoxic effects of dimethylnitrosamine in the rat. J Path Bact 91:605-608. |
| [33] | Martino PE, Diaz Gomez MI, Tamayo D, et al. 1988. Studies on the mechanism of the acute and carcinogenic effects of N-nitrosodimethylamine on mink liver. J Toxicol Environ Health 23:183-192. |
| [34] | Adeyemo, O.K., 2005. Haematological and histopathological effects of cassava mill effluent in Clarias gariepinus. Afr. J. Biomed. Res., 8: 179-183. |
| [35] | Wade JM, Omoregie E, Ezenwaka I. Toxicity of cassava (Manihot esculenta Crantz) effluent on the Nile tilapia, Oreochromis niloticus (L.) under laboratory conditions. J. Aquat. Sci., 2002; 17: 89-94. |
| [36] | Ivanova, E., T. Staikova and I. Velcheva, 2002. Mutagenic effect of water polluted with heavy metals and cyanides on Pisum sativum plant in vivo. J. Balkan Ecol., 3: 307-310. |
| [37] | Staykova TA, Ivanova EN, Velcheva IG. Cytogenetic effect of heavy-metal and cyanide in contaminated waters from the region of southwest Bulgaria. J. Cell Mol. Biol., 2005; 4: 41-46. |
| [38] | Seacat, A. M., P. J. Thomford, K. J. Hansen, G. W. Olsen, M. T. Case and J. L. Butenhoff. 2002. Subchronic toxicity studies on perfluorooctanesulfonate potassium salt in cynomolgus monkeys. Toxicol. Sci., 68, 249–264. |
| [39] | Seacat, A. M., P. J. Thomford, K. J. Hansen, L. A. Clemen and S. R. Eldridge. 2003. Sub-chronic dietary toxicity of potassium perfluorooctanesulfonate in rats. Toxicology, 183, 117–131. |
| [40] | Thibodeaux, J. R., R. G. Hanson, J. M. Rogers, B. E. Grey, B. D. Barbee, J. H. Richards, J. L. Butenhoff, L. A. Stevenson and C. Lan (2003): Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: Maternal and prenatal evaluations. Toxicol. Sci., 74, 369–381. |
| [41] | Luebker, D. J., M. T. Case, R. G. York, J. A. Moore, K. J. Hansen and J. L. Butenhoff. 2005a. Twogeneration reproduction and cross-foster studies of perfluorooctane sulfonate (PFOS) in rats. Toxicology, 215, 126–148. |
| [42] | Luebker, D. J., R. G. York, K. J. Hansen, J. A. Moore and J. L. Butenhoff. 2005b. Neonatal mortality from in utero exposure to perfluorooctanesulfonate (PFOS) in Sprague-Dawley rats: Doseresponse, and biochemical and pharamacokinetic parameters. Toxicology, 215, 149–169. |
| [43] | Du Y, Shi X, Yu K, Liu C, Zhou B. 2008. Chronic Effects of Waterborne PFOS Exposure on Growth, growth, survival and hepatotoxicity in zebrafish: a partial life-cycle test. Chemosphere 74, 723–729. |
| [44] | Agunbiade SO, Daramola OT, Anugweje KC, Onianwa O, Okonko IO. Hepatotoxic Effect Of Subacute Exposure Of Treated Carbanaceous Effluent On Mice. Cancer Biology 2012;2(2):15-22 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML