-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Frontiers in Science
p-ISSN: 2166-6083 e-ISSN: 2166-6113
2012; 2(5): 92-100
doi: 10.5923/j.fs.20120205.02
Spinning and Applications of Spider Silk
Kunal Singha , Subhankar Maity , Mrinal Singha
Department of Textile Engineering, Panipat Institute of Engineering & Technology, Harayana, India
Correspondence to: Kunal Singha , Department of Textile Engineering, Panipat Institute of Engineering & Technology, Harayana, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Spider silk is specially produced from the different species (sp.) of spider throughout the globe, specially the most fine and mechanically sound silk produced from nephila sp. The fibriller parallel model of spider silk gives higher mechanical properties with better Young’s modulus and lower stress than normal silk coming from the silk-moth. In this review work we dicuss about the collection, composition, spinning and molecular structure, mechanical properties with its application of the spider silk.
Keywords: Spider Silk, Nephila Sp., Fibriller Parallel Model, Young’s Modulus, Spinning, Mechanical Properties
Cite this paper: Kunal Singha , Subhankar Maity , Mrinal Singha , "Spinning and Applications of Spider Silk", Frontiers in Science, Vol. 2 No. 5, 2012, pp. 92-100. doi: 10.5923/j.fs.20120205.02.
Article Outline
1. Introduction
- Spider silk refers to a wide range of continuous filaments spun by the several species of lepidoptera & arthropoda. Spiders have six or seven sets of glands spun different fibers. There is different type of spider species; but the most common species of the spider which produced the spider dragline silk is Araneus diadematus[1, 2].There are various types of spider species available in the nature and their habitat, location, life cycle are also totally different. The web produced by them is different in color, strength, composition according to their lifestyle and food[3].The molecular composition in different silk can be as shown in table 1 and table 2. The glysine, alanine is the major amino acids presents in the spider silk. Due to the heavy benzene aromatic ring presents in these two amino acids the spider gives the higher mechanical strength than the normal silk obtained from the silk-moth cocoons[3,4]. The other important components are like side chains, polar heads which makes it strongly hydrophobic in nature. Generally the complex proteins presents in the spider silk is called as ‘spidroins’.The average diameter of major ampullate dragline silk spidroin 2.53 + 0.4 m. the virgin spider silk is infused within by mucopolysaccharide (highly viscous in nature, figure 3c) on the surface of the silk fibers so it is removed by toluene treatment for further spinning steps[13,14]. The SEM micrograph shows that toluene treated dragline silk gives a higher smooth and lustrous surface than the normal fiber with an abundant gummy part with a totally fuzzy surface (figure 3 a, b).
2. Macromolecular Structure of Silk Spidroin
- Major ampullate dragline silk is comprised of two proteins joined together via 3-5 disulfide bonds near their C-termini[12]; the two proteins which are found in spider silk are: spidroin 1 and spidroin 2.
2.1. Secondary Structure of Dragline Silk
- The crystalline structure of the beta (β) sheets is formed by the polar aligning of the protein molecules[15, 16].
3. Spider Orb Web
- The spider web where the spider excretes their saliva in the form of a web by their ampullatory gland is consisting some of special feature over its construction as follows[17];[a] Several spokes laid outward from a common origin.[b] ragline, minor ampullate, and viscid silks form the major portion of the orb web.[c] Dragline silk, in the form of mooring threads, framework and radial thread web dominate the web structure[18-20].
3.1. To Build up Their Web They Follow Pattern
- The spider generally builds their web in some stages as illustrated in figure 6. Initially the spider used to make a beam bridge between two supports and after then with the time they spin their web with the help of the excretion from their ampullatory gland[24].
3.2. Collection of spider silk
- [a] Naturally spun fibers are either retrieved from the spider web or from the safety line[21]. [b] Forcibly silk fibers are obtained by pulling the silk fibre from the spider at controlled speed.
3.3. Spinning of Spider Silk
- [a] Occur at very low temperature.[b] Used water as a solvent.[c] Needs three pairs of spinnerets –Anterior lateral, posterior lateral & posterior median.[d] Dragline silk protein is exits from anterior lateral spinneret[22].S[e] torage of spinning dope in Lumen of MA Gland.[f] MA spidroins are stored in the lumen of the gland in the presence of sodium and chloride ions, and during fiber spinning these ions are exchanged for potassium and phosphate ions[23-24].
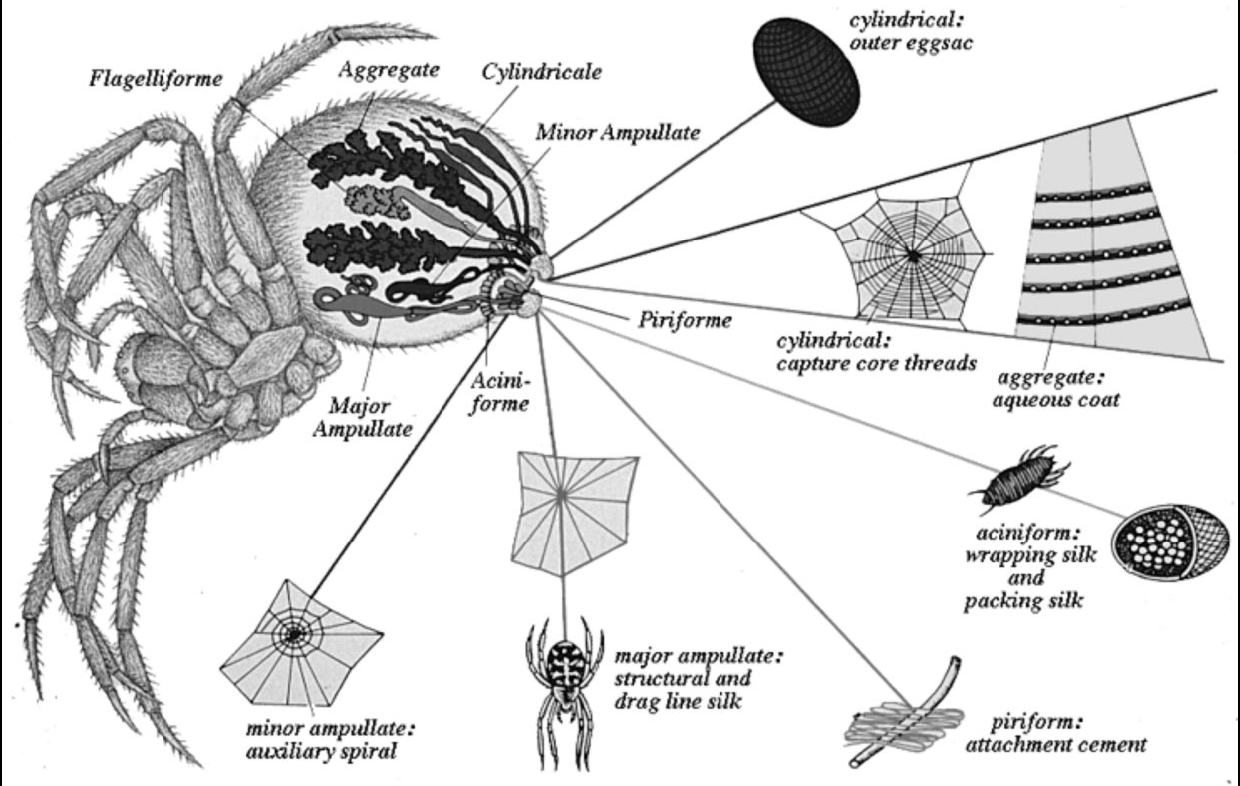 | Figure 1. The silk glands and threads of Araneus diadematus Spider |
 | Figure 2. Different species of the spider silk[3] |
|
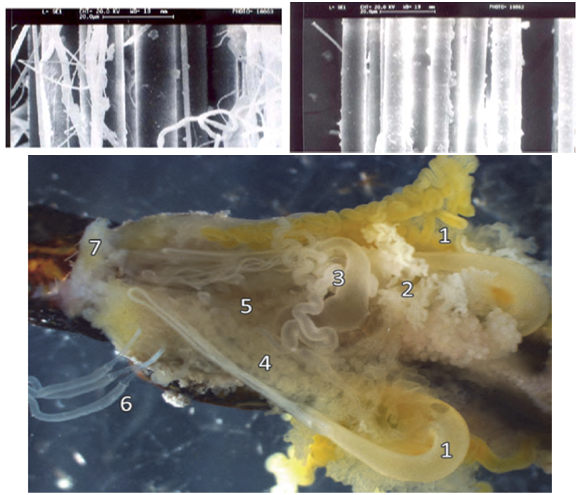 | Figure 3. (a) SEM micrograph of untreated dragline silk with gummy part (b) SEM micrograph of treated dragline silk with toluene (c) Microdissection of the silk-producing glands from the abdomen of N. clavipes indicates several specialized structures that manufacture silk fibroins. Numbers indicate each gland: 1 (top and bottom – major ampullate), 2 (flagelliform), 3 (minor ampullate), 4 (aggregate), 5 (aciniform), 6 (tubuliform) and 7 (pyriform)[11,12] |
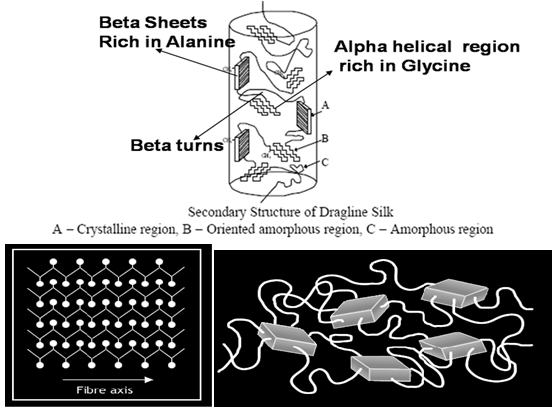 | Figure 4. Dragline structure of the molecular β- sheets composition of spider silk[16] |
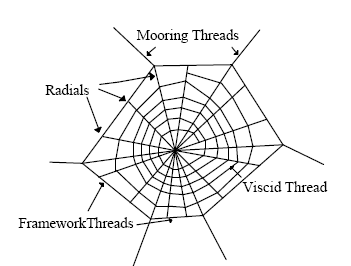 | Figure 5. Different parts of the spider silk web (orb web)[17] |
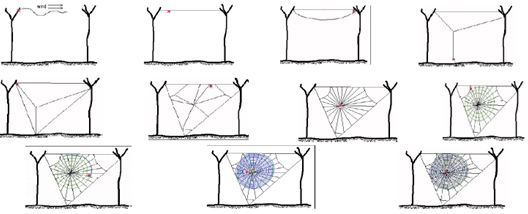 | Figure 6. Schematic illustration of different stages forming the web |
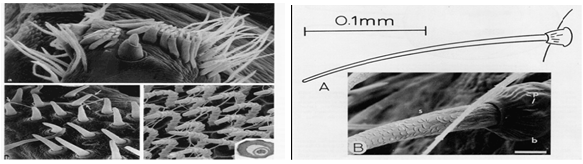 | Figure 7. Image of the spinneret of the Cribellate orb web weaver utoborus showing different types of spigots[23] |
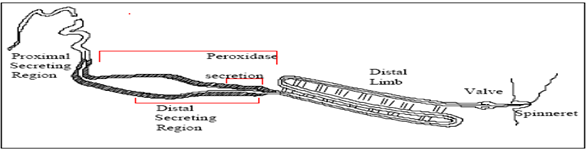 | Figure 8. Natural spinning system of dragline silk[24] |
3.4. Fiber for Mation During Spinning
- [a] Extension of molecules under stress enables them join with hydrogen bonds.[b] Give anti parallel beta conformation in final thread[26].[c] As silk protein molecules aggregate & crystallize, hydrophilic groups are hidden into anterior. [d] Surface becomes hydrophobic[29].[e] Facilitate loss of water from the surface of the extruded thread[25].[f] Water reabsorbed.[g] The effect of salt on protein aggregation (figure 9).[h] Low concentrations of salt improve the solubility of proteins (known as ‘salting in’), due to the formation of ion-rich hydration layers in the vicinity of charged and polar amino acid residues[27]. [i] High concentrations of salt, causing the protein to precipitate (known as ‘salting out’).[j] The effect of shear on protein aggregation: protein aggregation is increased in presence of shear, in both low and high concentrations of salt as shown in figure 10[25].
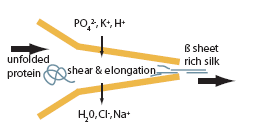 | Figure 9. Function of salt in protein separation in spider silk spinning[29] |
 | Figure 10. Effects of shear on orientation of spider silk fiber during spinning[25] |
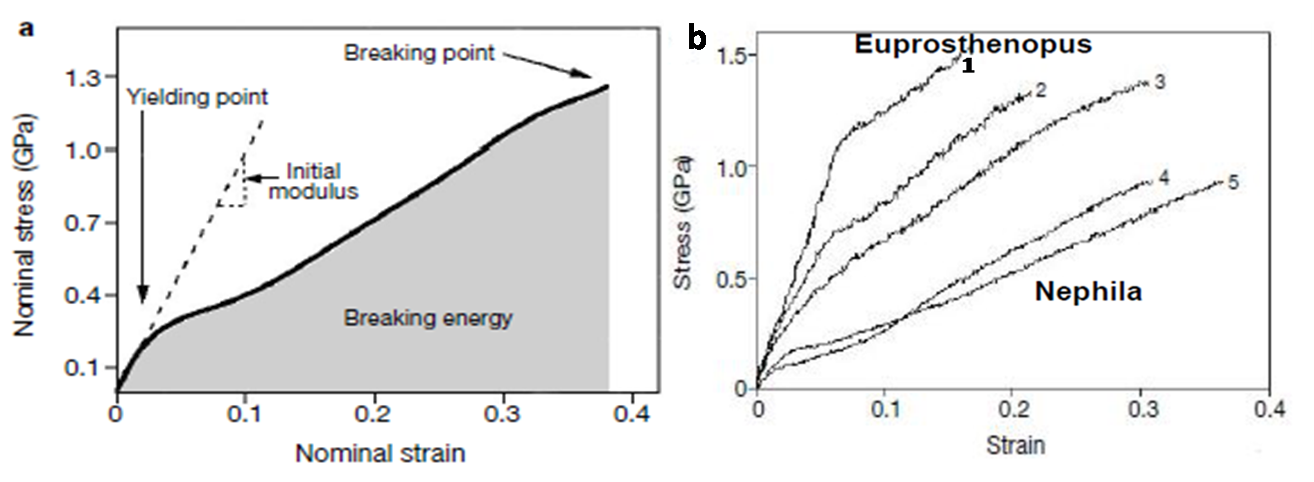 | Figure 11. (a) Stress-strain characteristics of the spider silk (b) for different sp. of spider[28] |
4. The Effect of Shear on Protein Aggregation
- [a] Aggregates formed after longer periods of exposure to shear were fibrous with dimensions of micrometer to mm scale. [b] At first we will not able to follow any stress-strain properties at very load, the response of the curve will show when the spider silk is being stretched t a quietly higher force.[c] Ratio of stress to strain provides the Young Modulus which is actually derived from slope of curve as shown in the figure 11 (a).[d] Young Modulus is required to measure the fiber stiffness.[e] Curve suddenly changes slope because of major structural transition in material[28].Stress Strain curve vary between same type of silk from different spiders like the Silk of Euprosthenopus stiffer require more force to break, less elastic, take up less energy than nephila sp. Silk as shown in figure 11 (b)[6].
4.1. Spinning Environment Control Material Properties of Final Silk Thread
- [a] Variability is due to differences in both the chemical composition of the dope & condition under which it spun.[b] Silk reeling speed & body temperature affect the diameter of silk thread & which influences force needed to break thread Action of solvents, Supercontraction as depicted in figure 12.[c]Spider silk shrink to half its original length & doubles in diameter (when in contact with water) called super contraction[22].[d]It involves a reversible uptake of solvent by silk.[f]Increase fiber extensibility but reduced stiffness.
4.2. Supercontraction
- [a] Consequence of hydrogen bond destruction by water molecules, result in molecular chain motion & disorientation.[b] Larger hydrogen bond destroyed larger shrinkage.[c] Super contracted in the presence of other NaSCN, urea, methanol., also take place in air (RH% of 90)[d] Can be re extended to their original length in same medium[29].
4.3. Strees-Strain Graph Of Supercontracted Fiber
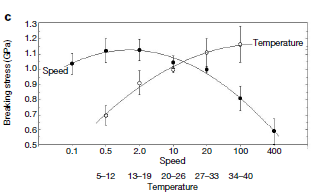 | Figure 12. Effects of temperature on mechanical properties of spider silk[22] |
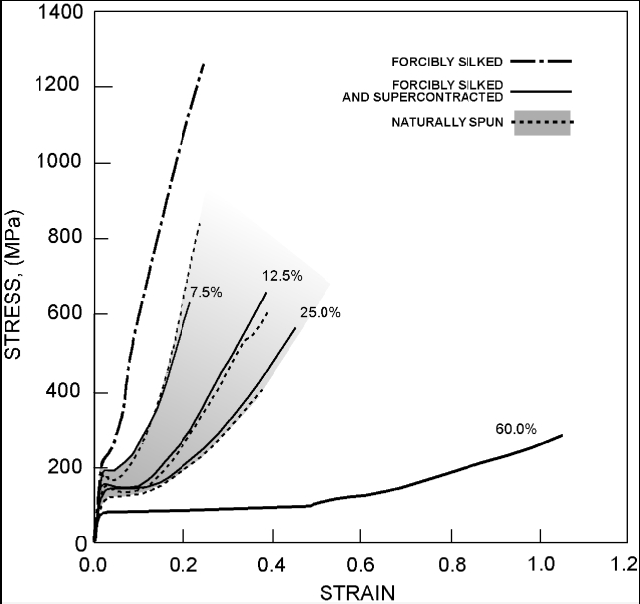 | Figure 13. Stress-strain properties of supercontracted fiber[29] |
5. Influence of The Controlled Supercontraction Length on the Stress–Strain Curves of Spider Silk
- [a] Forcibly silk fibers are stiffer than naturally spun ones.[b] There is a monotonous decrease in the stiffness of the fibre as supercontraction increases.[c] Supercontraction above 25% gives stress–strain curves that are more compliant than those of naturally spun fibers[24].
5.1. Significance of Controlled Super Contraction
- Supercontraction is introduced as a post spinning treatment to tailor final tensile properties of artificial silk fibers as it provides the opportunities to give the higher unidirectional force which forms the micro-domain in parallel combination fibriller model. The supercontracted solution of the polymer gives the heavy viscous nature with the higher intrinsic viscosity (IV) and it makes easy to stretching and drawing in further steps in a spinning line[8].
6. Problems with Spider Silk Generation
- [a] Spiders cannot be farmed like silkworms since they are cannibals and will simply eat each other if in close proximity [b] The silk produced is very fine so 400 spiders would be needed to produce only one square yard of cloth. [c] The silk also hardens when exposed to air which makes it difficult to work with[6-8].
7. Work Done in Genetic Engineering for The Production of The Excellent Quality Spider Silk
- Randolph V. Lewis, Professor of Molecular Biology at the University of Wyoming in Laramie Nexia Biotechnologies Inc in, Montreal, Canada had successfully developed a new type of spider species with a higher luster, mechanical stress over the normal silk by help of gene insertion into fungi and soya plants.
8. Applications of Spider Silk
- [a] Bullet-proof clothing [b] Wear-resistant lightweight clothing [c] Spider silk used as artificial muscle (Some spider silk contracts dramatically, up to 50 percent, when it gets wet. Researchers are applying this mechanism to create artificial muscles).[d] Ropes, nets, seat belts, parachutes[e] Rust-free panels on motor vehicles or boats [f] Biodegradable bottles [g] Bandages, surgical thread[1,29-30][h] Artificial tendons or ligaments, supports for weak blood vessels.
References
| [1] | D. Saravanan et al Spider Silk – Structure, Properties and Spinning, ,Journal of Textile and Apparel ,Technology and Management , Vol. 5, Issue 1, Winter 2006, 67-73. |
| [2] | Fritz Vollratha, Strength and structure of spiders’ silks, Reviews in Molecular Biotechnology 742000.67] 83 Review article. |
| [3] | Spider’s silk: Investigation of spinning process, web material and its properties, Rohit S. Gole and Prateek Kumar Department of Biological Sciences and Bioengineering, Indian Institute of Technology Kanpur, Kanpur-208016, India. |
| [4] | Riekel et al, Spider silk fibre extrusion: combined wide & small angle X- ray microdiffraction experiments,C. Riekel et al, International Journal of Biological Macromolecules, 29 (2001) 203-210. |
| [5] | Lukas Eisoldt, John G. Hardy, Markus Heim, Thomas R. Scheibel The role of salt and shear on the storage and assembly of spider silk proteins, Journal of Structural Biology (2010) Article in press. |
| [6] | Fritz Vollrath et al, Liquid crystalline spinning of Spider Silk, Nature, Vol. 410 (2001) 44-51. |
| [7] | Osnat et al, Spider & Mulberry silkworm silks as compatible biomaterials, Composites, Part B 38 (2007) 324-337. |
| [8] | J. Pe´rez-Rigueiro, M. Elices, G.V. Guinea Controlled supercontraction tailors the tensile behaviour of spider silk Polymer 44 (2003) 3733–3736. |
| [9] | Rammensee et al. Assembly mechanism of recombinant spider silk proteins PNAS, May 6 vol.105, (2008) no.18 6593. |
| [10] | Agapov, I., Pustovalova, O. L., Moisenovich, M. M., Bogush, V. G., Sokolova, O. S., Sevastyanov, V. I., et al. Three-dimensional scaffold made from recombinant spider silk protein for tissue engineering. Dokl Biochem Biophys, 426, (2009) 127-130. |
| [11] | Allmeling, C., Jokuszies, A., Reimers, K., Kall, S., & Vogt, P. M., Use of spider silk fibres as an innovative material in a biocompatible artificial nerve conduit. J Cell Mol Med, 10(3), (2006) 770-777. |
| [12] | Bini, E., Foo, C. W., Huang, J., Karageorgiou, V., Kitchel, B., & Kaplan, D. L., RGDfunctionalized bioengineered spider dragline silk biomaterial. Biomacromolecules, 7(11), (2006) 3139-3145. |
| [13] | Ando, Y., Okano, R., Nishida, K., Miyata, S., & Fukade, E., Piezoelectric and related properties of hydrated silk fibroin. Rep Prog Polymer Physics Japan, 23, (1980) 775-778. |
| [14] | Casem, M. L., Turner, D., & Houchin, K., Protein and amino acid composition of silks from the cob weaver, Latrodectus hesperus (black widow). Int. J. Biol. Macromol., 24(2-3), (1999) 103-108. |
| [15] | Askarieh, G., Hedhammar, M., Nordling, K., Saenz, A., Casals, C., Rising, A., Johansson, J., Knight, S., Self-assembly of spider silk proteins is controlled by a pHsensitive relay. Nature, 465(7295), (2010) 236-238. |
| [16] | Ayoub, N. A., Garb, J. E., Tinghitella, R. M., Collin, M. A., & Hayashi, C. Y., Blueprint for a high-performance biomaterial: full-length spider dragline silk genes. PLoS ONE, 2, (2007) e514-e519. |
| [17] | Blasingame, E., Tuton-Blasingame, T., Larkin, L., Falick, A. M., Zhao, L., Fong, J., Vaidyanathan, V., Visperas, A., Geurts, P., Xu, X., La Mattina, C., & Vierra, C., Pyriform spidroin 1, a novel member of the silk gene family that anchors dragline silk fibers in attachment discs of the black widow spider, Latrodectus hesperus. J Biol Chem, 284(42), (2009) 29097-29108. |
| [18] | Brooks, A. E., Nelson, S. R., Jones, J. A., Koenig, C., Hinman, M., Stricker, S., & Lewis, R., Distinct contributions of model MaSp1 and MaSp2 like peptides to the mechanical properties of synthetic major ampullate silk fibers as revealed in silico. Nanotechnol Sci Appl, 1, (2008) 9-16. |
| [19] | Dicko, C., Vollrath, F., & Kenney, J. M., Spider silk protein refolding is controlled by changing pH. Biomacromolecules, 5(3), (2004) 704-710. |
| [20] | Du, N., Liu, X. Y., Narayanan, J., Li, L., Lim, M. L., & Li, D., Design of superior spider silk: from nanostructure to mechanical properties. Biophys J, 91(12), (2006) 4528-4535. |
| [21] | Fahnestock, S. R., & Bedzyk, L. A., Production of synthetic spider dragline silk protein in Pichia pastoris. Appl. Microbiol. Biotechnol., 47(1), (1997) 33-39. |
| [22] | Casem, M. L., Tran, L. P., & Moore, A. M., Ultrastructure of the major ampullate gland of the black widow spider, Latrodectus hesperus. Tissue Cell, 34(6), (2002) 427-436. |
| [23] | Foelix, R., Biology of spiders. New York: Oxford University Press, Ed. I (1996). |
| [24] | Hu, X., Lawrence, B., Kohler, K., Falick, A. M., Moore, A. M., McMullen, E., Jones, P., Vierra, C., Araneoid egg case silk: a fibroin with novel ensemble repeat units from the black widow spider, Latrodectus hesperus. Biochemistry, 44(30), (2005) 10020-10027. |
| [25] | Chen, X., Knight, D. P., & Vollrath, F., Rheological characterization of Nephila spidroin solution. Biomacromolecules, 3(4), (2002) 644-648. |
| [26] | Craig, C. L., Riekel, C., Herberstein, M. E., Weber, R. S., Kaplan, D., & Pierce, N. E., Evidence for diet effects on the composition of silk proteins produced by spiders. Mol. Biol. Evol., 17(12), (2000) 1904-1913. |
| [27] | Hu, X., Vasanthavada, K., Kohler, K., McNary, S., Moore, A. M., & Vierra, C. A., Molecular mechanisms of spider silk. Cell Mol Life Sci, 63(17), (2006a) 1986-1999. |
| [28] | Hu, X., Kohler, K., Falick, A. M., Moore, A. M., Jones, P. R., & Vierra, C., Spider egg case core fibers: trimeric complexes assembled from TuSp1, ECP-1, and ECP-2. Biochemistry, 45(11), (2006b) 3506-3516. |
| [29] | Huemmerich, D., Helsen, C. W., Quedzuweit, S., Oschmann, J., Rudolph, R., & Scheibel, T., Primary structure elements of spider dragline silks and their contribution to protein solubility. Biochemistry, 43(42), (2008) 13604-13612. |
| [30] | Hu, X., Yuan, J., Wang, X., Vasanthavada, K., Falick, A. M., Jones, P. R., La Mattina, C. & Vierra, C., Analysis of aqueous glue coating proteins on the silk fibers of the cob weaver, Latrodectus hesperus. Biochemistry, 46(11), (2007) 3294-3303. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML