-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Frontiers in Science
p-ISSN: 2166-6083 e-ISSN: 2166-6113
2012; 2(3): 28-36
doi:10.5923/j.fs.20120203.03
Heavy Metal Pollution in Mithi River of Mumbai
Pravin U. Singare, Ravindra M. Mishra, Manisha P. Trivedi
Department of Chemistry, Bhavan’s College, Munshi Nagar, Andheri (West), Mumbai 400058, India
Correspondence to: Pravin U. Singare, Department of Chemistry, Bhavan’s College, Munshi Nagar, Andheri (West), Mumbai 400058, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The present study highlight the toxicity levels of different heavy metals in aquatic environment of one of the most polluted river of Mumbai- the Mithi River. Although the river has attracted tremendous attention after 26/7 flood in Mumbai, the pollution level of the river has remained neglected issue. The present study was performed for two assessment years 2009-10 and 2010-11 at three different sampling stations namely Airport, CST Kalina and BKC Taximen’s Colonyalong the flow of Mithi River. The results of present investigation indicates that the concentration levels of most of the heavy metals like Al, As, Cd, Cr, Hg, Ni, Pb, Sr and Mn obtained during the assessment year 2010-11 was higher than that obtained during 2009-10 by the factor of 1.4 to 5.7 µg/L. The environmental impact of these toxic heavy metals is discussed. The results point out the need of rational planning of pollution control strategies, so as to keep check on release of toxic heavy metals in the river. It is expected that the present study will be useful for rational planning of pollution control strategies and their prioritisation; to evaluate effectiveness of pollution control measures already is existence and to assess the nature and extent of pollution control needed.
Keywords: Heavy Metals, Water Pollution, Toxicity, Environmental Impact, Mithi River, Mumbai, India
Cite this paper: Pravin U. Singare, Ravindra M. Mishra, Manisha P. Trivedi, Heavy Metal Pollution in Mithi River of Mumbai, Frontiers in Science, Vol. 2 No. 3, 2012, pp. 28-36. doi: 10.5923/j.fs.20120203.03.
Article Outline
1. Introduction
- Heavy metals are considered very important and highly toxic pollutants in the various environmental departments. Ecotoxicologists and environmental scientists use the term “heavy metals” to refer to metals that have caused environmental problems. Heavy metals including both essential and non-essential elements have a particular significance in ecotoxicology, since they are highly persistent and all have the potential to be toxic to living organisms[1]. The metals which have been studied extensively the last decades are: Cd, Hg, Zn, Cu, Ni, Cr, Pb, Co, V, Ti, Fe, Mn, Ag and Sn. Some metals that have received more attention are Hg, Cd, and Pb, because of their highly toxic properties and their effects on the environment and the living organisms. Inputs of these toxic heavy metals to the environment as a result of anthropogenic activities is difficult to measure due to the very large natural inputs from the erosion or rocks, wind-blowing dusts, volcanic activity and forest fires. Atmospheric and river inputs, dredging spoil, direct discharges, industrial dumping and sewage sludge are some of the important contributors to metal pollution, which lead to the release of toxic heavy metals to the marine environment. Some metals enter the sea from the atmosphere, e.g. natural inputs of metals, such as Aluminium (Al) in wind-blowing dust of rocks and shales, and mercury (Hg) from volcanic activity. Lead (Pb) inputs in the atmosphere from industrial and vehicular exhaust are much greater than natural inputs. Some metals are deposited by gas exchange at the sea surface, by fallout of particles (dry deposition) or are scavenged from the air column by precipitation (rain) which is called wet deposition. The nature of metals depends on ore-bearing deposits in the catchment area and the discharge of human waste when the river passes through urban areas. Dredging of shipping channels produces large quantities of metal pollution. Much smaller quantities of metals are added to the sea by direct discharges of industrial and other waste and the dumping of sewage sludge[2]. Acid rain resulting from dissolved hydrogen sulphide, sulphur dioxide and oxides of nitrogen has contributed to alterations of soil and freshwater acidity. As a consequence there is an increase in the bioavailability of many heavy metals to freshwater biota[3].Metals are separated into the essentials and non-essentials in classes A and B, and in a borderline class[2].Class A metals: Calcium (Ca), Magnesium (Mg), Manganese (Mn), Potassium (K), Sodium (Na), Strontium (Sr) Class B metals: Cadmium (Cd), Copper (Cu), Mercury (Hg), Silver (Ag) Borderline metals: Zinc (Zn), Lead (Pb), Iron (Fe), Chromium (Cr), Cobalt (Co) Nickel (Ni), Arsenic (As), Vanadium (V), Tin (Sn).The world-wide emissions of metals to the atmosphere (thousands of tons per year) by natural sources is estimated as: Ni: 26, Pb: 19, Cu: 19, As: 7.8, Zn: 4, Cd: 1.0, Se: 0.4, (x103tons.Year-1). Whereas, from anthropogenic sources: Pb: 450, Zn: 320, Ni: 47, Cu: 56, As: 24, Cd: 7.5, Se: 1.1 (x103tons. Year-1). It is obvious from these numbers that Pb, Zn, Ni and Cu are the most important metal pollutants from human activities[4]. Heavy metal pollution of freshwater ecosystems has been shown to be extensive[5]. Rivers make a major contribution of metals in the marine environment and are considered as a dominant pathway for metals transport[6]. As a result heavy metals become significant pollutants of many riverine systems. The behavior of metals in natural waters is a function of the substrate sediment composition, the suspended sediment composition, and the water chemistry. During their transport, the heavy metals undergo numerous changes in their speciation due to dissolution, precipitation, sorption and complexation phenomena[7,8] which affect their behaviour and bioavailability[9]. Hence, heavy metals are sensitive indicators for monitoring changes in the aquatic environment.The preservation of aquatic resources for ecosystem and human health and well-being is a paramount concern worldwide and it has become evident that approaches to managing aquatic resources must be undertaken within the context of ecosystem dynamics in order that their exploitation for human uses remains sustainable[10]. If aquatic resources are not properly managed and aquatic ecosystems deteriorate, then human health and well-being may be compromised. Water quality monitoring for the detection of trends, impacts, and improvements is further complicated because the issues of concern and available resources are constantly changing[11]. Although it is not always possible to predict new and emerging threats to aquatic ecosystems, baseline water quality monitoring must be maintained to facilitate the early detection of such threats. The success of local, regional, and global efforts to curb rates of water quality degradation can only be measured if sufficient data are available that enable the tracking of trends over time and space. The problem of water quality degradation due to toxic heavy metals has begun to cause concern now in most of the major metropolitan cities in Maharashtra state of India and Mumbai is not an exception to it. The day by day deteriorating quality of water bodies in the country[12-22] has prompted us to carry the systematic and detail study of pollution due to toxic heavy metals in water of Mithi River, which due to rapid urbanisation and industrialisation is considered as one of the highly polluted river of Mumbai.
2. Materials and Methods
2.1. Area of Study
- The water sampling was done from three different sampling stations along the flow of Mithi River namely Airport (L1), CST Kalina (L2) and BKC Taximen’s Colony (L3). Airport site near Jari Mari area is thickly populated and has many small scale industries including scrap dealers. Previous short term study conducted by Maharashtra Pollution Control Board shows the presence of cyanide, consistent high COD, oil and grease found at this station indicating some chemical activity in that area[21]. Unauthorized encroachments by illegal industrial units, scrap dealers and oil mixing business at CST road near Kalina, have further resulted in discharge of solid waste, organic waste, industrial waste, heavy metals, oils and tar in the river. This sampling point is surrounded by many small scale industries including recyclers, barrel cleaners, workshops and other units. This area has thick density of population. Illegal activities like washing of oily drums have resulted in discharge of unauthorized hazardous waste which is carried out along the bank of this river. Development of Bandra-Kurla Complex has resulted in diversion and unnatural turn along the Mithi River at few places thereby affecting natural flow of the river and seriously affected the drainage. This part of the river is a dumping ground for garbage and it is reflected in higher values of suspended solids. The organic waste, sludge and garbage dumping has reduced the carrying capacity of the Mithi River. The solid wastes which is discharged in to the Mithi river from the surrounding illegal industries and the slums has resulted in sever water logging during 26/7 deluge in Mumbai. The map showing flow of Mithi River is shown in Figure 1.
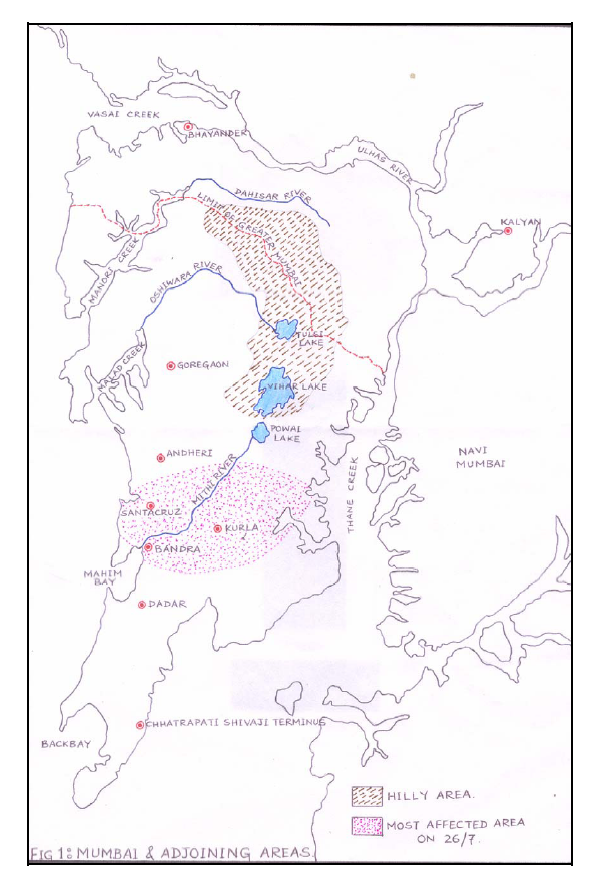 | Figure 1. Map showing flow of Mithi River in Mumbai |
2.2. Climatic Conditions
- The area is located along western Arabian cost of India from 18 deg. 53’ north to 19 deg. 16’ north latitude and from 72 deg. east to 72 deg. 59’ longitude. The area experiences tropical savanna climate. It receives heavy south west monsoon rainfall, measuring 2166 mm on an average every year. The temperature ranges from 16 deg. centigrade to 39 deg. centigrade with marginal changes between summer and winter months. Whereas relative humidity ranges between 54.5 to 85.5%.
2.3. Requirements
- The chemicals and reagent were used for analysis were of Analytical Reagent (AR) grade. The procedure for calculating the different parameters were conducted in the laboratory. The laboratory apparatus were acid soaked (nitric acid) before the analysis. After acid soaked, it is rinsed thoroughly with tap water and de-ionised distilled water to ensure complete removal of traces of cleaning reagents. The pipettes and burette were rinsed with solution before final use.
2.4. Water Sampling and Sample Preparation
- The water samples were collected randomly twice in a month in morning, afternoon and evening session from three different sampling stations along the flow of Mithi River. The samples were collected and subsequently analysed for a span of two years starting from October 2009 to September 2011. The sampling was done in three shifts i.e. morning shift between 07:00 a.m. to 09:00 a.m., afternoon shift between 02:00 p.m. to 04:00 p.m. and evening shift between 07:00 p.m. to 09:00 p.m. Polythene bottles of 2.5 L and 2.0 L were used to collect the grab water samples (number of samples collected, n = 19). The bottles were thoroughly cleaned with hydrochloric acid, washed with tape water to render free of acid, washed with distilled water twice, again rinsed with the water sample to be collected and then filled up the bottle with the sample leaving only a small air gap at the top. The sample bottles were stoppard and sealed with paraffin wax. Water samples (500 mL) were filtered using Whatman No. 41 (0.45 μm pore size) filter paper for estimation of dissolved metal content. Filtrate (500 mL) was preserved with 2 mL nitric acid to prevent the precipitation of metals. The samples were concentrated to tenfold on a water bath and subjected to nitric acid digestion using the microwave-assisted technique, setting pressure at 30 bars and power at 700 Watts[23,24]. About 400 mL of the sample was transformed into clean glass separating funnel in which 10 mL of 2% ammonium pyrrolidine dithiocarbamate, 4 mL of 0.5 M HCl and 10 mL of methyl isobutyl ketone (MIBK) are added[25]. The solution in separating funnel was shaken vigorously for 2 min and was left undisturbed for the phases to separate. The MIBK extract containing the desired metals was then diluted to give final volumes depending on the suspected level of the metals[26]. The sample solution was then aspirated into air acetylene flame in an atomic absorption spectrophotometer.
2.5. Heavy Metal Analysis by AAS Technique
- The analysis for the majority of the trace metals like aluminium (Al), cadmium (Cd), chromium (Cr), nickel (Ni), lead (Pb), strontium (Sr) and manganese (Mn) was done by Perkin Elmer ASS-280 Flame Atomic Absorption Spectrophotometer. Arsenic (As) was determined by hydride generation coupled with an atomic fluorescence detector, while mercury (Hg) was analysed with a cold-vapour atomic adsorption spectrophotometer. The calibration curves were prepared separately for all the metals by running different concentrations of standard solutions. A reagent blank sample was analyzed and subtracted from the samples to correct for reagent impurities and other sources of errors from the environment. Average values of three replicates were taken for each determination.
2.6. Quality Control/Assurance
- Water samples were collected in polythene bottles that were free from heavy metals and organics and well covered while transporting from field to the laboratory to avoid contamination from the environment. Reagent blanks were used in all analyses to check reagent impurities and other environmental contaminations during analyses. Analytical grade reagents were used for all analyses. All glassware used were soaked in appropriate dilute acids overnight and washed with teepol and rinsed with deionised water before use. Tools and work surfaces were carefully cleaned for each sample during grinding to avoid cross contamination. Replicate samples were analysed to check precision of the analytical method and instrument. To validate the analytical procedures used, the spike recovery test was conducted on some samples for Al, As, Cd, Cr, Ni, Pb, Sr, Mn and Hg.
3. Results and Discussion
- A number of elements are normally present in relatively low concentrations, usually less than a few mg/L, in conventional irrigation waters and are called trace elements. Heavy metals are a special group of trace elements which have been shown to create definite health hazards when taken up by plants. Under this group are included, Cr, Cd, Ni, Zn, Cu, Pb and Fe. These are called heavy metals because in their metallic form, their densities are greater than 4 g/cc. The experimental data on concentration (µg/L) of toxic heavy metals like Al, As, Cd, Cr, Hg, Ni, Pb, Sr and Mn in the water samples collected along sampling stations L1, L2 and L3 of Mithi River is presented in Table 1. The trend in average concentration of these metals at different sampling stations for two assessment years 2009-10 and 2010-11 is graphically represented in Figures 2-4. The effects of aluminium (Al) have drawn our attention, mainly due to the acidifying problems. Al may accumulate in plants and cause health problems for animals that consume these plants. The concentrations of Al appear to be highest in acidified aquatic environment[27]. In such aquatic environment the number of fish and amphibians is declining due to reactions of aluminium ions with proteins in the gills of fish and the embryo's of frogs[28].
|
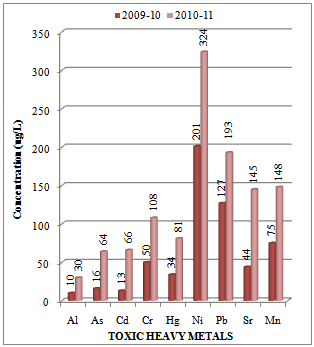 | Figure 2. Variation in average concentration values of different toxic heavy metals in water samples collected at sampling station L-1 of Mithi River during the assessment year 2009-10 and 2010-11 |
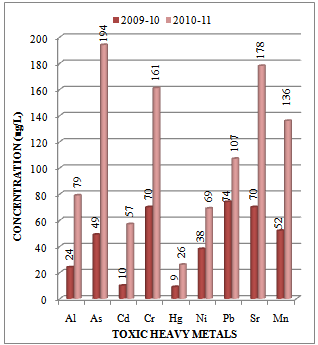 | Figure 3. Variation in average concentration values of different toxic heavy metals in water samples collected at sampling station L-2 of Mithi River during the assessment year 2009-10 and 2010-11 |
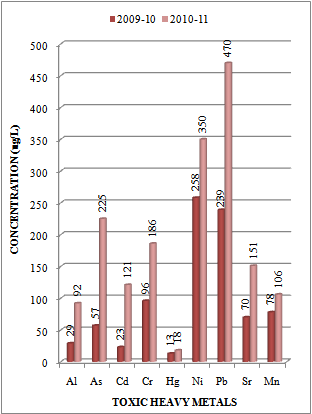 | Figure 4. Variation in average concentration values of different toxic heavy metals in water samples collected at sampling station L-3 of Mithi River during the assessment year 2009-10 and 2010-11 |
4. Conclusions
- Although in India the Central Pollution Control Board (CPCB) is responsible for restoration and maintaining the wholesomeness of aquatic resources under Water Prevention and Control of Pollution Act 1974 passed by Indian Parliament, it is expected that to maintained or restored the water quality at desired level it is important to have monitoring on regular basis. Also to address water quality related environmental problems, it is must to have accurate information and to know precisely what the problem is, where it is occurring, how serious it is, and what is causing it. Such information is necessary for determining cost effective and lasting solutions to water related problems. Hence it is expected that the regular water quality monitoring study as performed in the present investigation will help in understanding the water quality trends over a period of time and prioritising pollution control efforts. The present study will also be useful to assess assimilative capacity of a water body thereby reducing cost on pollution control; to assess the fitness of water for different uses.
ACKNOWLEDGEMENTS
- The authors are thankful to SAP Productions for developing and maintaining the paper template.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML