-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Frontiers in Science
p-ISSN: 2166-6083 e-ISSN: 2166-6113
2011; 1(1): 21-27
doi: 10.5923/j.fs.20110101.04
Spectroscopic Studies on Indian Portland Cement Hydrated with Distilled Water and Sea Water
D. Govindarajan 1, R. Gopalakrishnan 2
1Department of Physics, Annamalai University, AnnamalaiNagar, 608002, India
2Department of Physics, SRM University, SRM Nagar, 603203, India
Correspondence to: R. Gopalakrishnan , Department of Physics, SRM University, SRM Nagar, 603203, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The spectroscopicstudies have been carried out for Indian Portland cement hydrated with distilled and sea water in a water to cement ratio of 0.4. This study aims to analyze the effect of water on Portland cement. FTIR, DTA, XRD and EPR studies were used to characterize the hydration reaction of the cement pastes. Experimental results on setting time, compressive strength are also reported. The unreacted clinker phases and g-factors are calculated. The results indicate that sea water accelerates the cement hydration at early stage but retards in the latter stage of hydration.
Keywords: Spectroscopic Studies, Cement, Waters, Setting Time, Compressive Strength
Cite this paper: D. Govindarajan , R. Gopalakrishnan , "Spectroscopic Studies on Indian Portland Cement Hydrated with Distilled Water and Sea Water", Frontiers in Science, Vol. 1 No. 1, 2011, pp. 21-27. doi: 10.5923/j.fs.20110101.04.
Article Outline
1. Introduction
- Portland cement is a heterogeneous fine grained material consists of four main solid phases namely Tricalcium Silicate (C3S), Dicalcium Silicate (C2S), Tricalcium Aluminate (C3A), TetracalciumAlumino Ferrite (C4AF). During hydration of cement, Calcium Silicate Hydrate (C-S-H) gel and calcium hydroxide (Ca(OH)2) are formed from silicates phases and ettringite (AFt), monosulphate (AFm) are formed from aluminate phases[1]. C-S-H, a major hydration product is the main strength forming phase in the cement paste. Ca(OH)2 is a crystalline, isostructural with natural mineral of Portlandite. The cement hydration reactions are
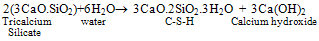 | (1) |
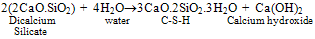 | (2) |
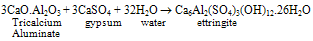 | (3) |
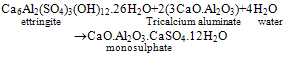 | (4) |
2. Materials and Methods
|
- A commercial Portland cement (PC) was used and subjected to chemical analysis using standard procedure suggested by ASTM and found the ingredients in percent as follows: CaO 63.32; SiO2 21.70; Al2O3 5.40; Fe2O3 3.40; MgO 2.09; MnO 0.12; SO3 2.10; Loss on ignition 0.79 and Insoluble residue 1.08. The distilled water and sea water was analyzed using standard procedure and given in Table 1.The pastes were prepared by mixing the cement with distilled water and sea water in water to cement ratio of 0.4. The samples were thoroughly mixed using glass rod for two minutes and then allowed to hydrate in air-tight plastic containers. The hydrations were stopped at different time intervals viz., 1 hour, 1 day, 1 week and 4 weeks by a consecutive soaking in acetone. To remove water content the hydrated cement pastes were oven-dried at 105℃ for 1 hour[12]. The samples hydrated for more than 1 day were cured properly. The dried samples were powdered using agate mortar for FTIR, DTA, XRD and EPR studies.The Fourier transform infrared measurements were recorded with a Nicolet-Avatar 360 model FTIR spectrometer using the KBr pellets technique. The Thermal curves of the OPC pastes were taken in a Perkin Elmer thermal analyzer. A total of 10-15 mg of the samples was heated in a platinum crucible in air atmosphere up to 1000℃ in a heating rate of 10℃/min. Compositional changes occur in the hydrated OPC pastes were identified by X-ray diffraction with CuKα radiation for Bragg’s angles between 5 and 70 with the scan rate of 0.1 to 120 degrees in 2θ/min. EPR spectra were recorded using JEOL JES-TE100 ESR Spectrometer operating at X-band frequencies, having a 100 KHz field modulation and DPPH is used as the standard reference for magnetic field correction. Setting time has been measured on DW and SW cement paste and compressive strength of the cement with DW and SW at different hydration times period were determined[13] and reported in Table 2.
3. Results and Discussion
3.1. FTIR Results
- The FTIR spectrum of the anhydrous Portland cement Fig. 1(a) shows a sharp band at 3630 cm-1 associated to O-H stretching vibrations of portlandite (Ca(OH)2) and the peaks at 3410 and 1610 cm-1 are correspond to stretching and bending modes of water of crystallization particularly from gypsum. The carbonates peak at 1425 cm-1, 717 cm-1 and 875 cm-1 are observed due to the reactions of atmospheric CO2with calcium hydroxide. The triplet bands appearing at 1100-1160 cm-1 are due to ν3 modes of
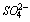 and the week bands at 659 cm-1 and 600 cm-1 are due to ν4 modes of
and the week bands at 659 cm-1 and 600 cm-1 are due to ν4 modes of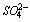 . The strong band at 919 cm-1 is due to Si-O asymmetric stretching vibration of C3S and/or C2S.Out of plane Si-O bending (ν4) and in-plane Si-O bending (ν2) are observed at 525 cm-1 and 455 cm-1 respectively. The band assignments are in good agreement with those reported in the previous studies[14-16].
. The strong band at 919 cm-1 is due to Si-O asymmetric stretching vibration of C3S and/or C2S.Out of plane Si-O bending (ν4) and in-plane Si-O bending (ν2) are observed at 525 cm-1 and 455 cm-1 respectively. The band assignments are in good agreement with those reported in the previous studies[14-16].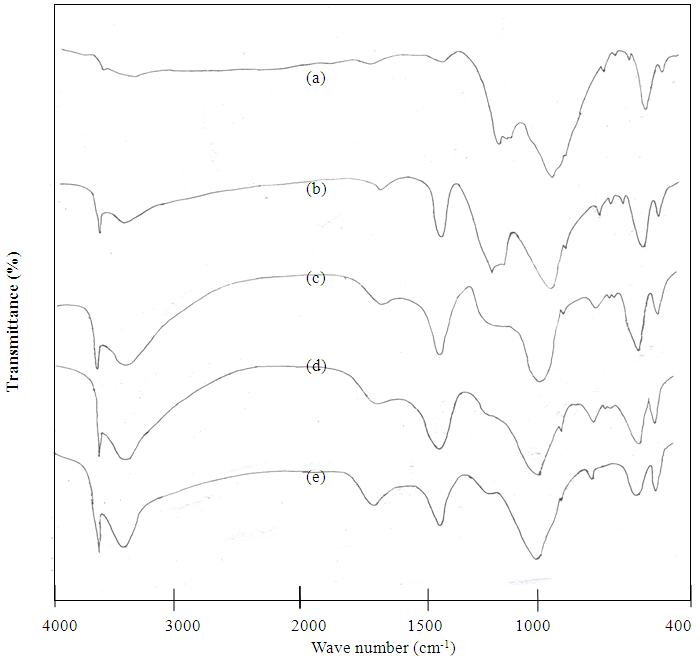 | Figure 1. FTIR spectra of (a) Anhydrous cement and Distilled water hydrated cement paste at (b) 1hour (c) 1 day (d) 1 week (e) 4 weeks |
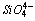 units in cement.For sea water treated cement pastes (Fig. 2), the same frequency assignment holds good as that of DW, but there is variation in intensities are observed. The shifting of ν3 Si-O band and variation in intensity of ν4 and ν2 Si-O bands are enhanced by SW are observed in early age. Up to 1 week, the intense sulfate band (
units in cement.For sea water treated cement pastes (Fig. 2), the same frequency assignment holds good as that of DW, but there is variation in intensities are observed. The shifting of ν3 Si-O band and variation in intensity of ν4 and ν2 Si-O bands are enhanced by SW are observed in early age. Up to 1 week, the intense sulfate band (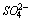 ) at 1100-1160 cm-1 are observed and it implies that SW enhance the AFt formation than DW. The observed variation in intensities of the band at 919-970 cm-1, 525 cm-1 and 455 cm-1 are higher and occur from early age onwards. This indicates that the initial reactions are faster due to the higher amount of chloride and sulfate ions present in the sea water[6,7]. At 4 weeks, the intensity of the band observed at 970 cm-1, 525 cm-1 and 455 cm-1 are less when compared to 1 week hydration. This may be due to disappearance of mineral contents in sea water. The above fact that increases the strength up to 1 week and slightly decreases at 4 weeks. Hence it is clear that SW accelerate the cement hydration at early stage but in latter stage slightly retarded.
) at 1100-1160 cm-1 are observed and it implies that SW enhance the AFt formation than DW. The observed variation in intensities of the band at 919-970 cm-1, 525 cm-1 and 455 cm-1 are higher and occur from early age onwards. This indicates that the initial reactions are faster due to the higher amount of chloride and sulfate ions present in the sea water[6,7]. At 4 weeks, the intensity of the band observed at 970 cm-1, 525 cm-1 and 455 cm-1 are less when compared to 1 week hydration. This may be due to disappearance of mineral contents in sea water. The above fact that increases the strength up to 1 week and slightly decreases at 4 weeks. Hence it is clear that SW accelerate the cement hydration at early stage but in latter stage slightly retarded.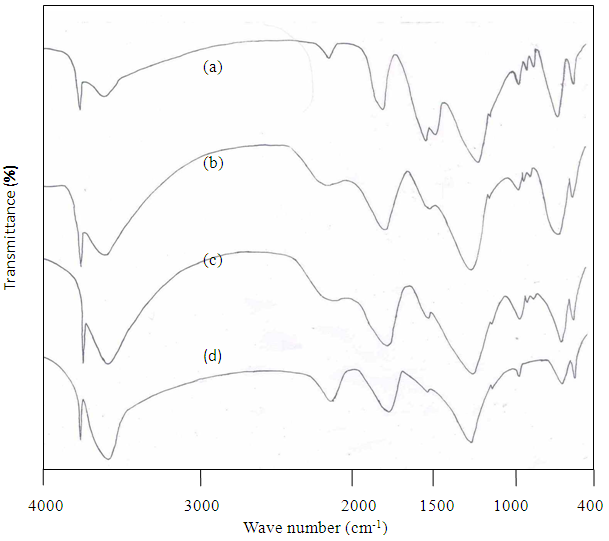 | Figure 2. FTIR spectra of Sea water hydrated cement paste at (a) 1hour (b) 1 day (c) 1 week (d) 4 weeks |
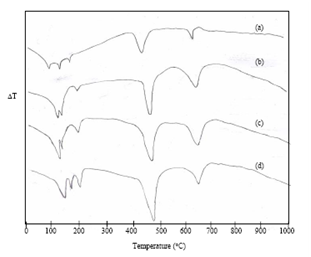 | Figure 3. DTA curves of Distilled water hydrated cement paste at (a) 1hour (b) 1 day (c) 1week (d) 4 weeks |
3.2. DTA Results
- Fig. 3 shows the DTA thermograms of OPC treated with DW at different hydration periods. At 1 hour hydrated cement paste, five endothermic peaks are observed. The peak at 98℃ and 160℃ are due to the presence of gypsum. The endothermic peak observed at 130℃ represents decomposition of ettringite. The fourth peak at 450℃ represents the decomposition of calcium hydroxide. The fifth endothermic peak at 670℃ is due to decomposition of calcium carbonate[18]. At 1 day, the peak at 98℃ and 160℃ are disappearing. This implies that the reaction of gypsum is almost exhausted. Further at 1 day, endothermic curve appears at around 115℃ and 180℃ indicates the formation of C-S-H and monosulphate. With progress of hydration, the endotherms due to C-S-H, monosulphate and Ca(OH)2 are increased in size (Fig. 3) are observed and also slightly shifting to a higher temperature. At 4 weeks, the intensity of the calcium carbonate endotherm is decreased. This is due to the reaction of CO2with C-S-H and Ca(OH)2 according to the following equation:[17]
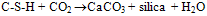 | (5) |
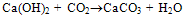 | (6) |
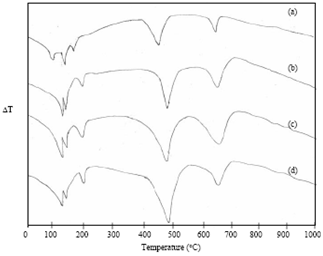 | Figure 4. DTA curves of Sea water hydrated cement paste at (a) 1hour (b) 1 day (c) 1 week (d) 4 weeks |
3.3. XRD Results
- Fig. 5(a) shows the XRD pattern of anhydrous cement. The spectrum indicated the presence of gypsum (2θ = 11.70), tricalcium silicate (2θ = 32.20, 51.50, 62.40), dicalcium silicate (2θ = 32.50, 56.15), tricalcium aluminate (2θ = 26.51), tetracalciumaluminoferrite (2θ = 44.10) and CaO (2θ = 37.60) respectively are coincide with previous reports[19-21].The XRD patterns of the cement hydrated with DW at 1 hour, 1 day, 1 week and 4 weeks are shown in Fig. 5(b-e).At 1 hour, ettringite (2θ = 9.10, 23.10, 41.25) and Ca(OH)2(2θ = 18.10, 33.57) crystallized phases are observed. At 1 day hydrationC-S-H (2θ = 29.50) and monosulphate (2θ = 56.64) phases are identified. The four clinker phases (C3S, C2S, C3A, C4AF) decreases with increasing hydration time and consequently C-S-H, Ca(OH)2 crystalline phase intensities are increases. The CaCO3(2θ = 38.68) peak is observed for all hydration time, due to the atmospheric carbon dioxide during grinding and preparation of samples[22].These results are confirmed through FTIR, DTA.
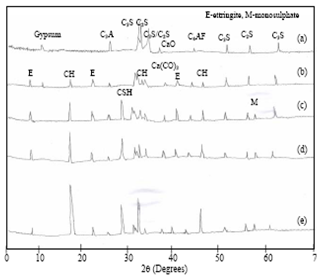 | Figure 5. XRD pattern of (a) Anhydrous cement and Distilled water hydrated cement paste at (b) 1hour (c) 1 day (d) 1 week (e) 4 weeks |
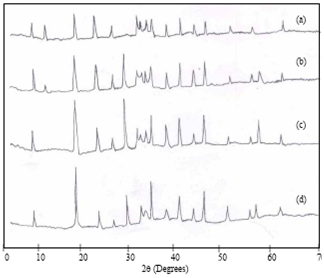 | Figure 6. XRD pattern of Sea water hydrated cement paste at (a) 1hour (b) 1 day (c) 1 week (d) 4 weeks |
| ||||||||||||||||||||||||||||||||
3.4. EPR Results
- The EPR spectra OPC paste mixing with DW and SW is shown in Figs. 7 and 8.For all samples the experimental parameters are the same and g-values are obtained from the equation g = hν/βB, where β is the Bohr magneton, h is the Planck’s constant, ν is the frequency and B is the center field at which the resonance occurred[24]. The g-value is main key parameter in identifying paramagnetic results in a particular symmetry. The g-values have been calculated for both Fe(III) and Mn(II) signals of cement pastes at different hydration period and are shown in Figs. 9 and 10(Table 4).
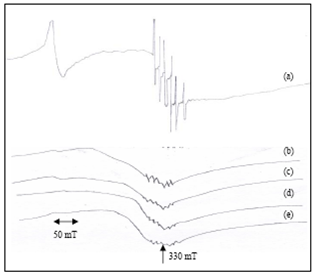 | Figure 7. EPR spectra of (a) Anhydrous cement (Frequency = 9.39624 GHz) and Distilled water hydrated cement paste at (b) 1hour (Frequency = 9.39723 GHz) (c) 1 day (Frequency = 9.37654 GHz) (d) 1 week (Frequency = 9.39465 GHz) (e) 4 weeks (Frequency = 9.39873 GHz) |
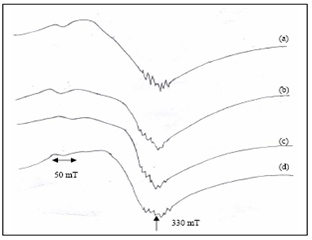 | Figure 8. EPR spectra of Sea water hydrated cement paste at (a) 1hour (Frequency = 9.39812 GHz) (b) 1 day (Frequency = 9.37731 GHz) (c) 1week (Frequency = 9.39654 GHz) (d) 4 weeks (Frequency = 9.39751 GHz) |
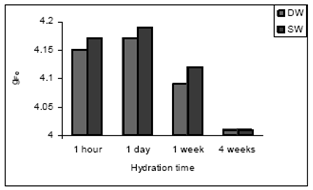 | Figure 9. gFe values vs hydration time of cement paste treated with DW and SW |
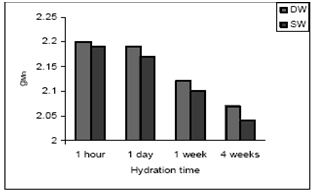 | Figure 10. gMn values vs hydration time of cement paste treated with DW and SW |
| |||||||||||||||||||||||||||||||||||||
3.5. Setting Time and Compressive Strength Results
- From the Table 2, the setting time of SW treated cement paste are shorter than those of DW treated cement paste. Moreover the sea water treated cement shows higher strength compared to distilled water treated cement up to 1 week and at 4 weeks the compressive strength are decreased. This is because that the following reason i) in the initial stage, sea water accelerates the reaction but in the latter stage the reaction is slower due to reduction in chloride and sulphate ions[6,7] ii) the reaction of C2S with sea water occurs earlier and hence in the later stage strength decreases when compared to DW treated samples. These results are very well agreed with FTIR, DTA and XRD.
4. Conclusions
- This paper describes spectroscopic studies on Indian Portland cement mixed with distilled and sea water. The following conclusion can be drawn from this study. 1. The results indicate that spectroscopic studies can be effectively used as a powerful tool in delineating the complexities of chemical reactions in cement hydration.2. The contents of mineral ions in sea water accelerate the Portland cement hydration especially at early ages. FTIR, DTA, XRD and EPR studies corroborated these results.3. EPR is a good tool to detect very small concentration s of Fe(III) and Mn(II) ions present in cement. g-factor values are confirmation of the well known accelerating effect of sea water in cement hydration.4. The effect of sea water in cement reduces the setting time, enhances the hydration and hence consecutive strength development at early stage but slightly retards in latter strength.5. The utility of sea water for preparing paste may be considered after studying about its long term reaction. Shrinkage properties, corrosion resistance and adhesion capacity are needed to be studied in the future.
References
| [1] | NEVILLEA M, BROOKS J J. Concrete Technology (Addison Wesley Longman Ltd)[B]. (1987) |
| [2] | WIELANDE, DÄHNR, VESPAM, LOTHENBACHB.Micro spectroscopicinvestigation of Al and S speciation in hardened cement paste[J].Cement Concrete Research, 2010, 40(6): 875-884 |
| [3] | BRUCKNERA, LUCKR, WIEKERW, WINKLER A, ANDREAE C, MEHNER H. Investigation of redox reactions proceeding during the hardening process of sulfide containing cement[J]. Cement Concrete Research, 1992, 22: 1161-1169 |
| [4] | CAIJUN S. Effect of properties of concrete on its electrical conductivity and the rapid chloride permeability test results[J]. Cement Concrete Research, 2004, 34: 537-545 |
| [5] | POON C, AZHAR S, ANSON M, WONG Y. Performance of metakaolin concrete at elevated temperatures[J].Cement Concrete Composites, 2004, 25:83-89. |
| [6] | BARATHAN S, GOVINDARAJAND, SIVAKUMAR G, RAGHUK. Microwave study of hydration of cement with different waters[J]. Indian Journal of Pure and Applied Physics, 2006, 44 : 334-338 |
| [7] | GHORAB H Y, HILALM S, KISHARE A. Effect of mixing and curing water on the behaviour of cement pastes and concrete Part. 1[J]. Cement Concrete Research,1989, 19: 868-878 |
| [8] | GANJIAN E and SADEGHI PH. Effect of magnesium and sulfate ions on durability of silicafume blended mixes exposed to the sea water. Cement Concrete Research, 2005, 35: 1332-1338 |
| [9] | KAUSHIK S.K and ISLAMS. Suitability of seawater for mixing structural concrete exposed to a marine environment[J]. Cement Concrete. Composites, 1995, 17; 177-184 |
| [10] | YIGITER H, YAZICI H and AYDIN S. Effect of cement type, water/cementratio and cement content on seawater resistance of concrete[J]. Building Environmen, 2007, 42; 1770-1777 |
| [11] | MANU SANTHANAM, COHEN M., OLEKJ. Differentiating sea water and ground water sulfate attack in Portland cement mortars[J]. Cement Concrete Research, 2006, 36(12), 2132-2137 |
| [12] | KOUROUNIS S, TSILIVIS S, TSAKIRIDIS P E, Papadimitriou G D, TSIBOUKI Z. Properties and hydration of blended cements with steelmaking slag[J]. Cement Concrete Research, 2007, 37; 815-822 |
| [13] | SHETTY M S, Concrete Technology (S. Chand and Co. Ltd, New Delhi)[B] (2004) |
| [14] | MOLLAHM Y A, KESMEZM, COCKE,D L. An FTIR and XPS investigation of the effects of carbonation on the stabilization/solidification of cement based systems Portland type V with Zinc[J]. Cement Concrete Research, 1993, 23; 773- 784 |
| [15] | PALOMO A, FERNANDEZ-JIMENEZ A, KOVALCHUK G,ORDONEZ L M, NARANJO M C[J].Chemical stability of cementitous materials based on metakaolin. Cement Concrete Research, 1999, 29(7): 997-1004 |
| [16] | OMOTOSO O E, IVEY D G, MIKULAR. Containment mechanism oftrivalent chromium in tricalcium silicate[J]. J. Haz. Mater, 1998,60: 1-28 |
| [17] | MOLLAH M Y A, PALTA P, THOMAS HESSR. Chemical andphysical effects of sodium lignosulfonate superplasticizer on the hydration of Portland cement and solidification/ stabilization consequences[J]. Cement Concrete Research, 1995, 25(3): 671-682 |
| [18] | ROJAS M F. Study of hydrated phases present in a metakaolin-lime system cured at 60℃ and 60 months[J]. Cement Concrete Research, 2006, 36;827-831 |
| [19] | TAYLORH W F, Cement Chemistry (Acadamic Press, Inc, New York) (1990) |
| [20] | HARCHAND K S, KUMAR R, SHARMAK, Mossbauer and X-Ray investigations of some Portland cements[J]. Cement Concrete Research, 1984, 14: 170-176 |
| [21] | MEDVESCEKS, GABROVSEKR, KEUCIC V, MEDENA. Hydration products in water suspension of Portland cement containing carbonates of various solubility[J]. Acta Chimica Slovonica 2006, 53; 172-179 |
| [22] | MORSY M S. Effect of temperature on hydration kinetics andstability of hydration phases of metakaolin-lime sludge- silica fumesystem[J]. Ceramics Silikaty, 2005, 49;225-229 |
| [23] | MONTGOMERYDM,SOLLARS C J, PARRY R, TARLING, S E,BARNESP,HENDERSONE. Treatement of organic contaminated industrial wastes using ement-based stabilizationa/solidification- I – microstructural analysis of cement – organic interactions[J]. Waste Management Research, 1991, 4: 113-11 |
| [24] | BRUCKNER A, LUCK R, WIEKER W, WINKLER A, ANDREAE C, MEHNER H. Investigation of redox reactions proceeding during the hardening process of sulfide containing cement[J]. Cement Concrete Research, 1992, 22: 1161-1169 |
| [25] | LUBOMIR LAPCIK and ZDENEK SIMEK Jr. Electron paramagnetic resonance study of dry cements[J]. Cement Concrete Research, 1996, 26(2); 237-242 |
| [26] | SAMBASIVA RAO P. Single crystal EPR study of Fe(III) doped magnesium potassium Tutton’s salt. Part 3 [J]. Spectra Chemica Acta Part A. 1996, 52; 1127-1134 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML