-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2023; 13(1): 6-14
doi:10.5923/j.fph.20231301.02
Received: Jul. 26, 2023; Accepted: Aug. 11, 2023; Published: Aug. 23, 2023

Multi Residue Analysis of Systemic Pesticide in Cocoa Beans from Some Farms in the Western North Region, Ghana
Alex Baah1, Boniface Yaayin2, Ruby Hanson2
1Department of Science, Holy Trinity Cathedral Senior High School, Accra, Ghana
2Department of Chemistry Education, University of Education, Winneba-Ghana, Winneba, Ghana
Correspondence to: Alex Baah, Department of Science, Holy Trinity Cathedral Senior High School, Accra, Ghana.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Multi-residue concentrations of systemic pesticides were analysed in cocoa beans sampled from the Western North region. This study's main objectives were to determine whether it was safe to consume cocoa products from the Western North Region by evaluating the residue concentrations of 11 systemic insecticides, 16 systemic fungicides, and 10 systemic herbicides in the sampled cocoa beans and comparing those concentrations to the European Union's Maximum Residue Limit (MRL) for cocoa beans. Ten cocoa farms and ten villages were selected by purposive sampling and simple random sampling respectively, from five Municipal and District Assemblies (MDAs) in the Western North Region. The sampled cocoa beans were examined using a QuEChERS Method MRM by LC-MS/MS that had been partially modified. 37 active compounds were examined. Only one pesticide, pirimiphos-methyl, was found, and its residue level of 0.01 mg/kg was within the EU's MRL for cocoa beans. All of the examined cocoa beans did not contain the other 36 pesticide residues. This suggests that cocoa beans from the Western North Region are of high quality, free of pesticide residues, and hence safe to eat because they provide no health risks to consumers. Additionally, the Region's cocoa sector will not be threatened by the export of cocoa beans to Europe as there were no pesticide residue concentrations exceeding the set MRL.
Keywords: Multi-Residue, Systemic, Pesticide, Cocoa Beans, Western North
Cite this paper: Alex Baah, Boniface Yaayin, Ruby Hanson, Multi Residue Analysis of Systemic Pesticide in Cocoa Beans from Some Farms in the Western North Region, Ghana, Food and Public Health, Vol. 13 No. 1, 2023, pp. 6-14. doi: 10.5923/j.fph.20231301.02.
Article Outline
1. Introduction
- Cocoa is a cash crop with particular interest for Ghana and for the global chocolate industry [1]. In Ghana, cocoa is cultivated on about 1.5 million hectares of land, owned by some 800,000 farm families in 10 out of the 16 regions in the country [2]. Over the years, cocoa has played very significant economic role for Ghana. These include being one of the highest contributors to Ghana’s Gross Domestic Product (GDP), employing about 60% of the country’s labour force [3], source of income for roughly 800,000 smallholder farm families in Ghana, providing food, employment, tax revenue and foreign exchange earnings for the country [1,4,5]. This is further supported by the report of Ghana COCOBOD which revealed that in Ghana, since independence, cocoa has been one of the biggest foreign exchange earners for Ghana, and the share of cocoa in Ghana’s GDP has been on the rise since the 2000s [6].Due to their unrivalled excellent quality, Ghanaian cocoa beans continue to command a significant premium on global commodities markets [6]. Further, according to Amoa-Awua, et al., Ghanaian cocoa beans are properly fermented, with uniform dark brown colour, sizes, and flavours, as well as a moisture content of 6 to 8% and little to no damaged beans or foreign material. This status has been assiduously upheld over time by the Quality Control Division (QCD), presently known as Quality Control Company Limited of COCOBOD [2].A study by [3] revealed that cocoa production in Ghana is threatened by insects, pests, and diseases, a situation that has led to a decline in cocoa production and a negative impact on the Ghanaian economy. This is true despite the economic significance of cocoa to the farmer and the country at large. This is consistent with earlier studies' findings that up to 40% of the world's annual cocoa production is lost to insects, pests, and diseases [7]. Similarly, according to [8], the main causes of West Africa's declining cocoa production in recent years have been pest and disease infestation. Moreover, Ghana's cocoa yields per hectare are far lower than those of other major cocoa-producing nations like Brazil, the Ivory Coast, and Cameroon [9]. According to [10], pests and diseases are a major factor in Ghana's low cocoa yields. Previous research has shown that the most reliable method for farmers to control cocoa pests and diseases and preserve the excellent quality of cocoa beans is by applying pesticides [11,12]. According to [13], a "pesticide" is any substance that is used to manage a pest at any point during the production, storage, or transportation of a crop. According to how or when they operate, all pesticides can be categorized as either having a contact or systemic mode of action [14]. Pesticides that control a pest as a result of contact are known as contact pesticides. When sprayed directly or when they come into contact with surfaces treated with a residual contact pesticide, insects, fungi, or weeds are killed. Contrarily, the pesticides under study (systemic pesticides) are those that are absorbed by plants or animals before spreading to other organs through untreated tissues. According to [14], systemic insecticides, fungicides, or herbicides spread throughout treated plants and kill specific insects, fungi, or weeds.However, because of the extensive use and various application rates, there is a risk of crop and environmental contamination when pesticides are used in large quantities. According to previous researches, sprayed pesticides in cocoa fields may infiltrate and persist in soils, water bodies, and cocoa beans for several months or years following treatment [6,10,15,16,17]. The biological environment and the natural environment are both challenged by the application of pesticides. According to a study by [18], when pesticide residues are ingested by plant roots and leaves from soils, air, and other nutrient solutions, they have the potential to be hazardous to plants, their products, and pollute the food chain. A study by [18] reported that exposure to pesticides through food and water can affect thyroid function, lower male sperm counts, birth defects, an increased risk of testicular cancer, immune system problems, endocrine disruptions, cancers, immunotoxicity, and neurobehavioral and developmental disorders. Similar to this, some researchers asserted that the increasing concentration of these pesticide residues in food may cause major health risks if they are not digested by the body and build up in the soft tissues [19,20].The Codex Alimentarius Commission (CODEX), the World Health Organization (WHO), and other designated authorities have prescribed tolerance Maximum Residue Limits (MRLs), Acceptable Daily Intake (ADI), and No Observable Adverse Effect Levels (NOAELs) for various pesticides in food (including cocoa beans), soil, and drinking water due to risks associated with consuming foods found to contain pesticide residues above the approved limits [15].The export of cocoa beans from Ghana, in particular, to its important trading partners in Europe and Japan, is subject to potential rejections if prohibited elements are identified in the product or at levels beyond the MRLs due to the strong implementation of the MRLs harmonization [15]. The livelihood of smallholder farmers will be threatened if cocoa beans are rejected due to a high level of forbidden pesticide residue concentrations, which will aggravate unemployment and poverty as well as the nation's ability to earn money abroad.Unfortunately, systemic pesticide residue knowledge in tropical foods, such as cocoa beans, and of actual sources of contamination remains low. Although numerous pesticides, including neonicotinoids, some organophosphorous, some pyrethroids, some fungicides, and herbicides with systemic action, have been used extensively in Ghana and other West African countries for several years to produce cocoa and other crops, it is still unknown how much of these chemicals are present in cocoa beans. A large portion of the literature's current understanding of systemic pesticide residues has been limited to honey, honey bees, avocados, and other fatty fruits and vegetables [21,22,23]. While there has been some research on pesticide residues in cocoa beans from Ghana, it has mainly focused on other classes of contact-action pesticides, such as carbamates, organochlorines, and organophosphates, which have been less frequently used in cocoa production in recent years, especially in Ghana [6,10,24]. To address this lacuna in literature, this study sampled fresh cocoa beans from the farms, fermented and dried them, and used a partially modified QuEChERS Method MRM by LC-MS/MS procedure in the extraction, purification/clean-up, and quantification of the 37 residues of systemic pesticides to assess the levels of systemic pesticides in them. Unlike previous studies in Ghana that sampled cocoa beans from the harbour and the depot offices. This current study has made it easier to identify the source of cocoa bean contamination and determine whether or not cocoa farmers apply pesticides carelessly, and also whether or not it is safe to consume products made from cocoa beans from these cocoa farms in accordance with WHO standards. Additionally, the Western North region was chosen for this study because it is one of the areas that produces the most cocoa in the nation [2,25], yet there is little information available on the region's residue concentrations [17,26]. Therefore, a multi-residue study of systemic pesticide concentrations in cocoa beans from the Western North Region was necessary in light of findings from literature.
2. Materials and Methods
2.1. Study Area
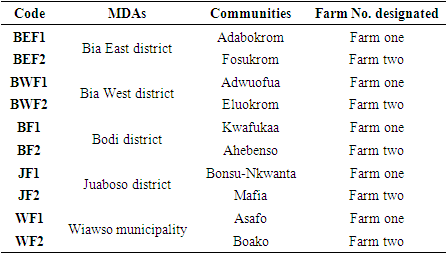
- The Western North Region served as the study's location. The area is one of Ghana's six newly established regions as of February 15, 2019. Out of Ghana's existing Western Region, this new region was created. It is located between latitude 6.227937°, or 6° 13' 41", and longitude -2.823489°, or 2° 49' 25", in the northern hemisphere. 10,079 km2 is the total area of the region's territory. The region is bordered on the west by the Comoe District of the Ivory Coast Republic, on the southeast by the Central Region, on the southwest by the Western Region, and on the north by the Ashanti, Ahafo, Bono East, and Bono regions. Three Municipal District Assemblies (MDAs) and six District Assemblies (DAs) make up the region. These are Aowin, Wiawso, Bibiani / Anhwiawso/Bekwai, Bia East, Bia West, Bodi, Juaboso, Sefwi Akontombra and Suaman respectively. Sefwi Wiawso is the administrative capital of the region.The Western North Region has year-round highs between 25°C and 30°C with moderate to heavy rainfall patterns between 1200 mm and 1780 mm per year [27]. It is located in the tropical rainforest climate zone with it peaks in June-July and September-October, it has double maxima features. The relative humidity is high, ranging from roughly 90% at night to 75% during the day. The region's rainfall pattern is distinct and ideal for agricultural pursuits [27]. There are two lengthy wet seasons followed by a brief dry season.The region's primary industry for employment is agriculture. The cultivation of food (such as plantains, cassava, cocoyam, rice, maize, fruits, vegetables, etc.) and cash crops (including cocoa), raising cattle, mining, and subsistence fishing are the main economic activities in the area [28]. The primary exportable goods include cereals, bauxite, gold, bauxite ore, cocoa, and timber. Figure 1 depicts the nine Municipal and District Assemblies in the Western North Region.
 | Figure 1. Map of Western North region showing the administrative divisions |
2.2. Sampling Technique and Sample Size
- In order to gather samples, five of the nine Municipal District Assemblies (MDAs) in the area were purposively chosen. They were Bodi, Juaboso, Wiawso, Bia East, and Bia West. These MDAs were chosen, as they were noted for the high cocoa production in the region and for the close proximity of these assemblies to one another. From each of the districts above that were chosen for the study, two communities (total of 10 communities) were picked at random. Then, one farm was purposefully chosen among the sampled communities. The following eligibility requirements were used to choose the farms in the communities:1. Farm age (at least eight years old and no older than twenty years) and history of pesticide use (at least five years). 2. The farmers' willingness to allow researchers to sample some of their chocolate beans. Samples of cocoa beans were gathered from March to May, 2022. Table 1 lists the cocoa farms that were chosen from among the ten communities in the area.
|
2.3. Sampling Design and Preparation of Samples of Cocoa Beans
- Two 80 × 80-meter quadrats were marked in each of the ten cocoa plantations. About five cocoa trees were chosen at random for each quadrat. A random sample of 10–20 mature, ripe, and fresh cocoa pods were collected. The cocoa beans were cracked open, fermented for seven days, and then sun-dried for an additional seven days. Foreign materials were hand-picked out after the beans had been air dried. In order to create a composite sample, the dried cocoa beans from each quadrat were bulked together. One kilogram samples were then taken and transported to the Ghana Standards Authority Pesticide Residue Laboratory in Accra for the analysis of pesticide residues.Each of the labelled fermented dried cocoa bean samples from each farm was ground into a fine powder (homogenized) at the Ghana Standards Authority Pesticide Residue Laboratory in Accra. The powder was then collected into a new sample plastic bag and re-labelled appropriately to form each individual analytical sample for each farm, weighing 1 kg. The mill was properly cleaned with a brush after each sample was ground. Before the analytical sample was collected into a new, marked sample plastic bag, a few grams of the following sample to be processed were powdered and discarded to prevent cross contamination.
2.4. Sample Extraction and Clean-up
- The QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) SPE (Solid-Phase Extraction) methodology was used in the analysis of the sampled cocoa beans. The QuEChERS procedure is usually a two-stage process consisting of sample extraction followed by dispersive SPE as enumerated below: Extraction: The cocoa samples were homogenized to maximize the available surface area of the samples for better extraction efficiencies. About 10g±0.1g of the comminuted homogenous sample was weighed into a 50 mL centrifuge tube. After this, 15 mL acetonitrile was added and vortex for 1 minute. The sample was then centrifuged for 5 minutes at 3500 rpm.Extract purification: Dispersive Solid Phase Extraction (SPE) - The second stage of the QuEChERS method uses dispersive SPE, which involves transferring a portion of the acetonitrile extract to a clean-up tube containing a combination of sorbents for removal of unwanted sample components. Approximately 6 mL aliquot of the extract was transferred into a 15 mL centrifuge tube which contains 150 mg PSA, 150 mg C-18 and 1 g magnesium sulphate. Magnesium sulphate ensures that, upon addition of acetonitrile, a phase separation is induced between water and organic solvent, with the pesticides of interest being extracted into the organic phase. The tube was closed and vortexed for 1 minute and then centrifuged for 5 min. at 3500 rpm. 1 mL of the purified extract was transferred into a 2 mL standard opening vial for quantitation by LC MS/MS.
2.5. Laboratory Requirements
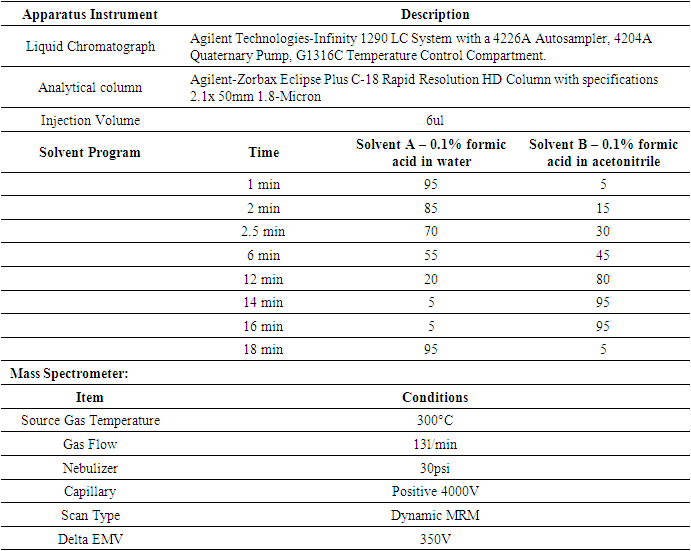
- The individual certified reference standards, acetamiprid, clothianidin, imidacloprid, thiamethoxam, acephate, allethrin, dimethoate, methamidophos, monocrotophos, pirimiphos-methyl, sulfoxaflor, boscalid, carbendazim, difenoconazole, dimethomorph, fluazinam, fluopyram, kresoxim-methyl, metalaxyl, prochloraz, pyrimethanil, spiroxamine, tebuconazole, tebufenozide, triadimefon, triadimenol and trifloxystrobin, aclonifen, ametryn, atrazine, bensufuron-methyl, bromacil, metolachlor, nicosulfuron, propaquizafop, terbutryn, and topramezone used for the identification and quantification were provided by the pesticide residue laboratory of the Ghana Standard Authority (GSA). These were obtained from the appropriate chemical stores and stored in the freezer to minimize degradation. The laboratory apparatus and materials used for the analysis are outlined in Table 2.
|
2.6. LC–MS/MS Instrumentation
- On an Agilent Technologies-Infinity 1290 LC System with a 4226A Autosampler, 4204A Quaternary Pump, and G1316C Temperature Control Compartment, chromatographic separation of analytes was carried out. Mobile phases A and B were composed of 95% acetonitrile in 5 mM ammonium acetate and 5 mM ammonium acetate, respectively. The gradient was carried out as follows for 16 minutes at 300 mL min 1: In 8 minutes, 10% B grew linearly to 100%; this value was maintained for 2 minutes; it then declined back to 10% B in 1 minute; and this value was kept for the entire 5-minute equilibration period. The injection has a 5 mL volume.The electrospray ionization (ESI) capability of the mass spectrometer was utilized. With multiple reaction monitoring (MRM) of the two most intense precursor-product ion transitions for each analyte, one used for quantification and the other for confirmation, the MS determination of all analytes was carried out in positive mode. Source Gas Temperature of 300°C, Gas Flow of 13l/min, Nebulizer Pressure of 30psi, Capillary Positive Voltage of 4000V, Scan Type of Dynamic MRM, and Delta EMV Voltage of 350V were the source characteristics used. With the help of the Analyst program (version 1.6.2), data were processed. Analytes were quantified from matrix-matched standard curves using peak area and a weighting of 1/x.
2.7. Quality Control and Quality Assurance
- To guarantee the accuracy of the results, proper quality assurance procedures and safety measures were performed. All foreign objects were manually picked out of the cocoa beans when being dried. By doing this, it was made sure that the 1 kg of pure cocoa beans were devoid of any impurities that would affect the sample weight and the analysis that followed. The zip-lock bags that were used were clean, dried, and free of any contaminants, pollutants, or dirt. The samples were also treated with care to eliminate any outside effects that can compromise their integrity and, as a result, taint them. The glassware was thoroughly cleaned, and the reagents were of analytical quality. All of the glassware that would be used for the extraction and cleaning of pesticide residues was thoroughly cleaned. After properly rinsing them with analytical grade acetone and distilled water, they were dried in a 150°C oven overnight. The glass items were then taken out of the oven, given time to cool, and placed in dust-free cabinets for storage. The entire investigation was conducted with deionized water. Repeated analyses of the samples against internationally certified/standard reference material (SRM-1570) from the National Institute of Standard and Technology were utilized to validate the analytical process. Additionally, the analysis of solvent blanks, procedural matrix blank duplicate samples, and correct calibration of all laboratory equipment all contributed to the assurance of the quality of pesticide residues. All reagents used in the analysis were subjected to the same extraction techniques before being tested for interference-causing compounds. Samples from all series were analysed in duplicates.
2.8. Ethical Consideration
- In this study, respecting both scientific integrity and the rights of the cocoa farmers where the study was done are ethical considerations. Before gathering samples of cocoa beans from the fields of the cocoa farmers, permission was obtained in order to uphold the ethical standards of the study. Most significantly, these farmers' privacy and anonymity were protected. Furthermore, the participants were not forced to take part in this study and were free to withdraw at any time. Therefore, their consent was sought and they willingly participated in the study.
2.9. Data Analysis
- Microsoft Excel 2019 was used to generate the mean scores for the 37 systemic pesticide residue concentrations. The mean score was computed for the two farms selected from each Municipal or District Assembly in the Western North region. Pesticides whose concentrations were not detected in the analysed cocoa beans samples were given a mean of ND, which means Not Detected.
3. Results and Discussion
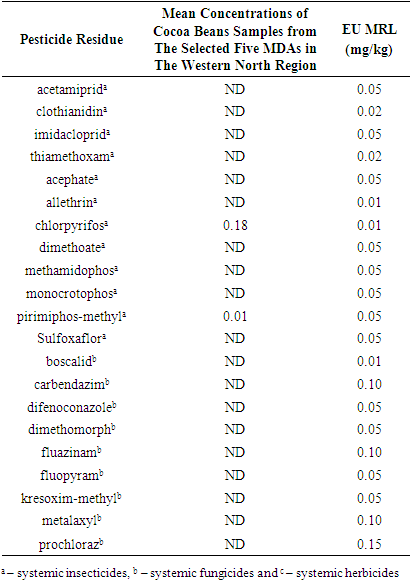
- This study analysed multiple residues of three categories of systemic pesticides, namely; systemic insecticide, systemic fungicide and systemic herbicide in cocoa beans from ten selected cocoa farms across five Municipal and District Assemblies (MDAs) within the Western North Region, Ghana. Eleven systemic insecticides, namely: acetamiprid, clothianidin, imidacloprid, thiamethoxam, acephate, allethrin, dimethoate, methamidophos, monocrotophos, pirimiphos-methyl, and sulfoxaflor. Five of these insecticides belonged to the neonicotinoids class, namely; acetamiprid, clothianidin, imidacloprid, thiamethoxam and sulfoxaflor. Currently, the neonicotinoids are the approved insecticides for cocoa in Ghana by the COCOBOD [10,15,16,25]. Five out of the 11 systemic insecticides analysed belonged to the organophosphorous class, and they were acephate, dimethoate, methamidophos, monocrotophos, and pirimiphos-methyl. Though these organophosphorous are not currently approved by the COCOBOD to control cocoa insects, previous studies still found their use and existence in cocoa beans from Ghana [15,16,24]. Allethrin belongs to the synthetic pyrethroid chemical class of insecticides and its use is unapproved in Ghana for the control of cocoa pests [25].Sixteen systemic fungicides: boscalid, carbendazim, difenoconazole, dimethomorph, fluazinam, fluopyram, kresoxim-methyl, metalaxyl, prochloraz, pyrimethanil, spiroxamine, tebuconazole, tebufenozide, triadimefon, triadimenol and trifloxystrobin. Among them, only dimethomorph, fluazinam and metalaxyl are the approved cocoa systemic fungicides in Ghana whiles the remainder are unapproved by the COCOBOD.Ten systemic herbicides analysed were: aclonifen, ametryn, atrazine, bensufuron-methyl, bromacil, metolachlor, nicosulfuron, propaquizafop, terbutryn, and topramezone. Currently, there are no approved herbicides for controlling weeds in cocoa farms by the Ghana COCOCBOD [25]. As a result, farmers are left with their own discretion to purchase any herbicide in the agrochemical market to control weeds in their farms. Personal observation and conversation with some cocoa farmers revealed that, the herbicide they often purchase to control weeds in their farms have systemic action. This is because systemic herbicides kill the entire plant by spreading gradually throughout its vascular system, from either foliar application down through the plant, or soil application, up towards the leave. Table 3 presents the mean concentrations of cocoa beans samples from the selected MDAs in the Western North Region.
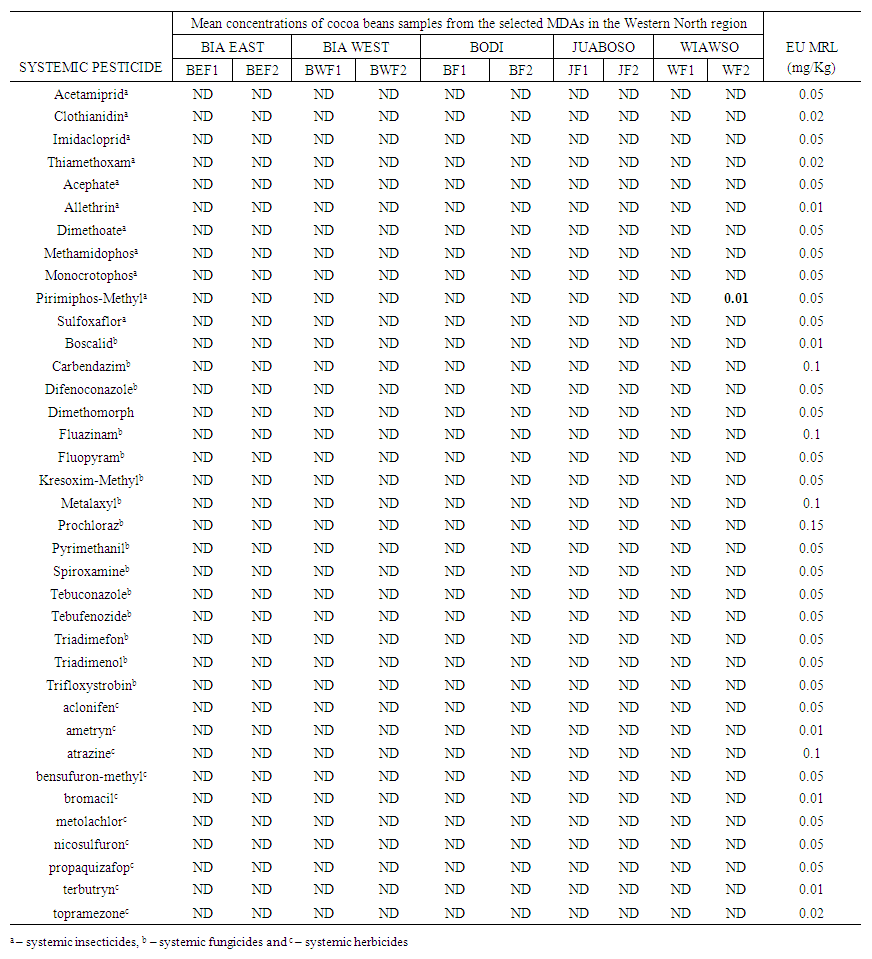 | Table 3. Mean concentrations of cocoa beans samples from the selected MDAs in the Western North Region |
3.1. Systemic Insecticides
- Out of the 11 systemic insecticides that were analysed, only pirimiphos-methyl was detected. Pirimiphos-methyl was detected in only one of the samples analysed. The residue concentration of Pirimiphos-methyl in the cocoa beans sampled from Boako (WF2) in the Wiawso Municipal was found to be 0.01 mg/kg. This concentration is within the approved limits of residue concentrations in cocoa beans by the European Union, as the EUs MRL for Pirimiphos-methyl was 0.05 mg/kg. Pirimiphos-methyl is one of the active ingredients in Cocostar (one of the insecticides approved by COCOBOD for use by cocoa farmers). In the late 1990s, imidacloprid (Confidor 200SL) and a cocktail of pirimiphos-methyl and bifenthrin under the trade name Axtellic Talstar were introduced to gradually replace lindane and propoxur as evidence began to emerge that resistance was being built against them cocoa insects [15]. Since then, pirimiphos-methyl has been used by cocoa farmers, instead of propoxur and lindane, and its presence has been detected in cocoa beans sampled and analysed from different study area in the country. The concentrations of pirimiphos-methyl recorded at all the study sites with detectable residues were below the European Union (EU) MRL of 0.05 mg/kg for cocoa beans [15,24]. The detection of this pesticide in cocoa beans samples in both current and previous studies suggests that pirimiphos-methyl was used by cocoa farmers for the control of cocoa insects, and farmers have been following the recommended dosage during its usage.The other 10 systemic insecticides analysed were not detected in all the sampled cocoa beans from the five MDAs in the Western North Region. These were acetamiprid, clothianidin, imidacloprid, thiamethoxam, acephate, allethrin, dimethoate, methamidophos, monocrotophos, and sulfoxaflor. This implies that either these pesticides are not used by farmers in the Western North Region or its residue level is still not detectable in the cocoa samples collected.Although this current study did not detect the presence of imidacloprid and thiamethoxam in all the cocoa beans sampled and analysed, some previous studies detected their presence in varying degrees ranging from not detected to 0.005 mg/kg residue in cocoa beans sampled from the cocoa shells, depots and ports [10,15,16]. This could be attributed to the fact that samples may comprise beans from multiple farms and multiple farmers in the various towns and villages.
3.2. Systemic Fungicides
- In Ghana, the fungicides approved by COCOBOD for use by cocoa farmers are Ridomil (Metalaxyl cuprous oxide), Nordox (Cuprous oxide), Funguran (Cuprous Hydroxide), Champion (Cuprous Hydroxide), Kocide (Cupric Hyroxide), Fungikill (Cupric Hydroxide + Metalaxyl), Metalm (cuprous Oxide + Metalaxyl), Banjo Forte 400 SC (Dimethomorph + Fluazinam), Forum R (Dimethomorph + Copper hydroxide), Sidalco Defender (Dicopper chloride trihydroxide, SC), Agro Comment 72WP, Fantic (Benalaxyl M + copper (I) oxide), Fantic (Benalaxyl M + copper (I) oxide), Vamos 500SC (Fluazinam), Royal Cop 50WP (Copper (II) hydroxide) and Delco 75WP (Copper (I) oxide) (Adjinah & Opoku, 2010; Boamah, 2021). However, a study by Okoffo (2016) revealed that 75% of farmers indicated their use of other pesticides that are not approved by COCOBOD for cocoa production. Most of the approved fungicides by the COCOBOD are non-systemic in action except those that have metalaxyl, dimethomorph and fluazinam as part of their active ingredients. This current study, therefore, analysed the presence of some systemic pesticides often used by farmers whether approved or not approved by the COCOBOD. The 16 systemic fungicides analysed were boscalid, carbendazim, difenoconazole, dimethomorph, fluazinam, fluopyram, kresoxim- methyl, metalaxyl, prochloraz, pyrimethanil, spiroxamine, tebuconazole, tebufenozide, triadimefon, triadimenol and trifloxystrobin. There was no detection for all the 16 systemic fungicides analysed in the sampled cocoa beans from the Western North Region. The use of systemic fungicide to control black pod disease is on the decrease due to the mild nature of the disease in cocoa farms in recent times. The non-detection of any of the systemic fungicide residues analysed suggests that it is either the farmers have stopped using it to control black pod disease (Phytophthora megakarya) in their farms so their presence has diminished in the whole cocoa beans sampled and analysed or farmers have been following the prescribed procedures for their use, hence the accumulated residue concentrations in the whole cocoa beans were not detectable.
3.3. Systemic Herbicides
- Aclonifen, ametryn, atrazine, bensufuron-methyl, bromacil, metolachlor, nicosulfuron, propaquizafop, terbutryn, and topramezone were all not detectable in the sampled cocoa beans. This means that farmers have been applying the recommended dosage of herbicides in the control of weeds in their farms or better still cocoa farmers have been relying on the indigenous but safer way of clearing weeds in their farms. This was evident during the sample collection as most of the cocoa farmers had clear weeds in their farms using machete. When probed further, the farmers argued that clearing weeds in their farms manually (using machete/cutlass) enabled them to turn the fallen and dried cocoa leaves which help to improve soil aeration. Thus, it is likely that majority of cocoa farmers in the Western North region still control weeds by clearing them with machetes / cutlasses rather than relying on herbicides.The non-detection of the systemic herbicides could also be attributed to the fact that these herbicides exert their effects on the weeds with relatively low stability in the treated environment. This assertion is supported by [29] who opined that in order to ensure that the newer generation of herbicides do not linger on after accomplishing their task, research is directed towards synthesizing biodegradable compounds. Thus, the ideal herbicide is one that degrades to harmless chemicals after it performs its function and therefore does not persist in nature.
3.4. Comparison of the Pesticides Residues Limits in the Cocoa Beans from the Region against European Union Standards for Maximum Residue Limits: Safety and Potential Health Risk Issues
- Table 4 compares the mean concentrations of the pesticide residues in the cocoa samples from the selected communities in the Western North Region to the European Union’s Maximum Residue Limits (MRL).
|
4. Conclusions
- There was minimal representation of systemic insecticide residues in the analysed cocoa beans sampled from the Western North region, Ghana. This minimal representation of pirimiphos methyl detected was within the acceptable range. There was no representation of both systemic fungicides and systemic herbicides in the analysed cocoa beans sampled from the region. The researchers aver that the Western North Region produces high-quality, pesticide-residue-free cocoa beans that are safe for consumption and offer no health risks to consumers. This suggests that the local farmers, who deserve praise, have followed the advised processes and dose while using pesticides on their crops. Additionally, the cocoa beans examined in This study region will not offer any serious threat to the cocoa business as far as shipments to Europe are concerned, taking levels of pesticide residues in fermented dry cocoa beans against the European (EU) commission standards on pesticide residues into consideration.
ACKNOWLEDGMENTS
- The authors are grateful to the cocoa farmers in the Western North region whose farms were used for this study. We are also to the Ghana Standard Authority for using their pesticide residue laboratory in analysing the samples.
Conflict of Interest
- The authors have no conflict of interest to declare.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML