-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2023; 13(1): 1-5
doi:10.5923/j.fph.20231301.01
Received: Jul. 5, 2023; Accepted: Aug. 7, 2023; Published: Aug. 12, 2023

The Functional Properties and Nutritional Profile of Flour made from Two Selected Plantain Varieties (Musa balbisiana and Musa accuminata)
Aderonke O. Mosuro 1, Ifeoluwa O. Bodunde 1, Mayowa F. Alade 1, Adeola A. Akinyele 2, Abisola O. Fawole 1
1Department of Human Nutrition & Dietetics, Lead City University, Ibadan, Nigeria
2Department of Human Nutrition and Dietetics, Federal Polytechnic, Ede, Nigeria
Correspondence to: Aderonke O. Mosuro , Department of Human Nutrition & Dietetics, Lead City University, Ibadan, Nigeria.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Africa is rich in variety of staple foods. The need to improve the available nutrient information on staple foods according to the specific variety cannot be overlooked to improve the existing food composition tables. Plantain is an economically viable agricultural produce. Its potential to be made into different food products and food forms increases this characteristic. The functional properties and nutritional profile of flours made from two selected plantain varieties (musa balbisiana and musa accuminata) were determined. Unripe plantains were purchased, washed, peeled, chopped into smaller chips, and sundried until a crispy form was observed. The chips were ground into powder and used for analysis. Functional properties and nutritional profile of the plantain varieties were determined. For functional properties, Musa balbisiana flour had higher (p<0.050) water absorption (78.00±0.00), oil absorption (69.00±0.00) and swelling capacity (10.42±0.00) than Musa accuminata. In contrary, Musa accuminata flour had higher solubility index (13.67±0.58) and bulk density (0.74±0.02). Also, Musa accuminata had higher (p<0.05) dry matter, ash, fibre, and protein (5.54±0.51%, 3.31±0.31%, 3.77±0.12 and 5.84±0.51, respectively) while Musa balbisiana had higher (p<0.05) fat and carbohydrate contents (2.33±0.58 and 83.14±0.36, respectively). Similarly, Musa balbisiana had higher macro-mineral contents (p<0.05) while chromium, copper, and vitamins B2, B3, and B6 contents were similar for both samples (p>0.05). In general, this study showed that flour made from the two varieties of plantain, although with varying nutritional profiles, are both nutrient-rich and can be used to ensure food and nutrition security.
Keywords: Plantain flour, Proximate composition, Functional properties, Food security, Unripe plantain
Cite this paper: Aderonke O. Mosuro , Ifeoluwa O. Bodunde , Mayowa F. Alade , Adeola A. Akinyele , Abisola O. Fawole , The Functional Properties and Nutritional Profile of Flour made from Two Selected Plantain Varieties (Musa balbisiana and Musa accuminata), Food and Public Health, Vol. 13 No. 1, 2023, pp. 1-5. doi: 10.5923/j.fph.20231301.01.
Article Outline
1. Introduction
- Plantains (Musa paradisiaca) are an inexpensive source of calories, and essential minerals such as potassium [1]. Plantain ranks third in production among starchy staples after cassava and yam and Nigeria is the world's top plantain producer, producing 2.5 million metric tons [2,3]. The diversity in the plantain crop results from a variety of cultivars. Many are hybrids derived from the cross of two wild species, Musa acuminata and Musa balbisiana. The currently accepted scientific name for all such crosses is Musa paradisiaca [4]. Plantains in their ripe and unripe forms are consumed in many various ways in Nigeria. They can be boiled, roasted, fried and ground into powder for the preparation of several delicacies. Consumption of unripe plantain is of particular interest due to its perceived nutritional and health associated benefits. Earlier studies [5,6,7] had reported the health benefits of plantains. Despite the high patronage and usage of plantain as staple food, very few information are available on the nutritional composition and functional properties in literature [8]. The nutrient and chemical properties of a food product are a major determinant of the food product’s health benefit [9]. The functional properties are characteristics that determine the response of nutrients in food samples during storage, processing, and preparation. Their responses determine the quality and acceptability of the food samples [10]. The water and oil absorption capacity, swelling capacity, bulk density, and solubility index are important properties that influence the use of starchy staples such as plantain [11]. An understanding of the nutritional and functional profile of plantain flour may be of benefit to the food industry especially in the formulation of new food products and may also find wider usage in the bakery and nutraceutical industries. This will address the gaps in existing food composition tables, improve available nutrient information on staple foods according to the specific food varieties and improve clinical nutrition practice and diet therapy.Hence this study was designed to add to existing literature on the proximate composition of flours from two varieties of plantain and also provide further knowledge on their functional properties.
2. Materials and Methods
2.1. Raw Material Procurement and Sample Preparation
- Two varieties of unripe plantain (Musa balbisiana and Musa accuminata) which are staples in the West and Central African diet, especially Nigeria, were purchased from Oluode market in Osogbo, Osun State. The unripe plantain fruits were washed in clean water and dried with paper towels before peeling and slicing. The peels were discarded and the pulps were sliced, arranged on porous trays and sundried until they were crispy. Dried samples were milled using Silver crest multifunction blender (SC-1589_Germany) into powder and stored in air tight containers for analysis.
2.2. Functional Properties Determination
- The swelling index, water/oil absorption capacity, solubility index, and bulk density (BD), were determined.Swelling index was determined according to Okaka and Potter [12]. 100 ml graduated cylinder was filled with the sample to 10 ml mark. The distilled water was added to give a total volume of 50 ml. The top of the graduated cylinder was tightly covered and mixed by inverting the cylinder. The suspension was inverted again after 2 min and left to stand for a further 8 min and the volume occupied by the sample was taken after the 8th min. The water absorption capacity of the flours was determined by the method of Sosulski et al. [13]. One gram of sample was mixed with 10 mL distilled water and allowed to stand at ambient temperature (30 ± 2°C) for 30 min, then centrifuged for 30 min at 3000 rpm or 2000 × g. Water absorption was examined as percentage water bound per gram of flour.Solubility was determined by dissolving one (1) g of flour in 10 ml of distilled water contained in a centrifuge tube. This mixture was stirred for 30 minutes with a stirrer and then kept in a water bath at 37°C for 30 minutes. It was then centrifuged at 5000 rpm for 15 minutes and dried at 105°C to a constant mass and weighed [14].The oil absorption capacity was determined [13]. One gram of sample was mixed with 10 ml soybean oil (Sp. Gravity 0.9092) and allowed to stand at ambient temperature (30 ± 2°C) for 30 min, then centrifuged for 30 min at 300 rpm or 2000 × g. Oil absorption was examined as percentage oil bound per gram of flour. The volume of 100g of the flour was measured in a measuring cylinder (250 ml) after tapping the cylinder on a wooden plank until no visible decrease in volume was noticed, and based on the weight and volume, the apparent (bulk) density was calculated [15].
2.3. Proximate Composition Determination
- The moisture, ash, protein, fat, and crude fibre were determined by the methods of AOAC [16]. Carbohydrate was determined by difference as 100 – (% moisture + % ash + % lipid + % crude protein).
2.4. Mineral and Vitamin Composition Determination
- Micronutrient contents such as potassium and sodium were assayed by flame spectrophotometry [17] while calcium, magnesium, and phosphorus were analyzed using atomic absorption spectrophotometry (Hot lab AA320N automatic flame graphite furnace double beam). Ascorbic acid assay was determined as described by Ndayambaje et al [18] and Beta-carotene was determined using the methods by James [19].
2.5. Statistical Analysis
- All data obtained were statistically analyzed using analysis of variance (ANOVA) to determine significant differences at5% level of significance using SPSS v24.0. The means were separated using the Duncan Multiple Range test. All data were expressed as mean± standard deviation of triplicate values.
3. Results and Discussion
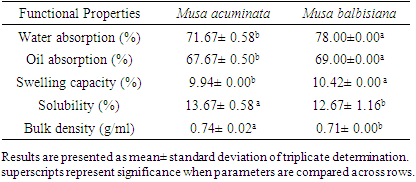
3.1. Functional Properties of the Samples
- The functional properties of the both plantain flour samples are shown in Table 1. Musa balbisiana had asignificantly (p<0.05) higher water absorption capacity (78.00± 0.00%), oil absorption capacity (69.00± 0.00%), and swelling capacity (10.42± 0.00%) than found in Musa accuminata (71.67± 0.58%, 67.67± 0.5%, 9.94± 0.00% respectively). Water absorption capacity is the property that determines how much water a flour can absorb in order to rise and improve yield and consistency during food processing [20]. It also gives the organoleptic properties that determine the uniqueness and consumer acceptability of the food product [21]. The water absorption capacity is usually dependent on the starch and non-starchy component of the flour and indicates the suitability of flour in baking [20]. The water absorption capacity from this study is lower compared to 130.00±1.00 and 196 ml/100g reported for unripe plantain flour sun-dried [20] and oven dried respectively [22]. Oil is important in the flavour retention and mouth feel of a food sample. The oil absorption of plantain flour samples is 3 times lower compared to results from Arinola et al. [20] and Osundahunsi [22] but similar to the values for the six cultivars studied by Honfo et al. in Benin [23].
|
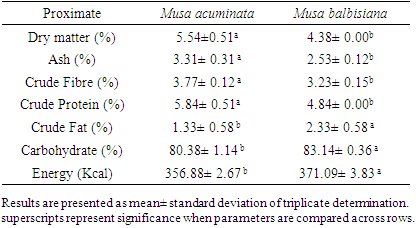
3.2. Proximate Composition of Plantain Samples
- Table 2 shows the proximate composition of the plantain flour samples. There were variations in the proximate composition of the plantain varieties. Musa accuminata contained a dry matter of 5.54±0.51% which was higher (P<0.05) than 4.38± 0.00 found in Musa balbisiana. The ash content for Musa accuminata was 3.31±0.31% compared with a lower (P<0.05) 2.53± 0.12 found in Musa balbisiana. This is higher than was found in the six cultivars from Benin [23], and in soy-plantain composite [28], but lower compared to oven-dried and sun-dried unripe plantain flour from Benue State, Nigeria [24]. The crude fiber of 3.77± 0.12% found in Musa accuminata was higher (P<0.05) than 3.23± 0.15% found in Musa balbisiana. However, these results were lower compared to the oven-dried and sun-dried plantain flours from Benue, Nigeria [29]. The protein content was significantly different (P<0.05) with 5.84± 0.51% and 4.38±0.00% recorded in Musa accuminata. and Musa balbisiana respectively. These results were higher compared to findings from Benue State [29] and fermented unripe and ripe plantain [30] but lower in soy-plantain flour [28]. Crude fat contents that were recorded in both samples were similar with 1.33± 0.58% and 2.33± 0.58% for Musa accuminata and Musa balbisiana, respectively. This was similar to the findings of Yarkwan and Uvir [29], and [30] in 72 hours fermented ripe and unripe plantain flour. Musa balbisiana contained 83.14±0.36% of carbohydrate which was higher than (P<0.05) 80.38± 1.14% in Musa accuminata. The energy content for Musa balbisiana 371.09±3.83% was significantly higher (P<0.05) than 356.88± 2.67 found in Musa accuminata.
|
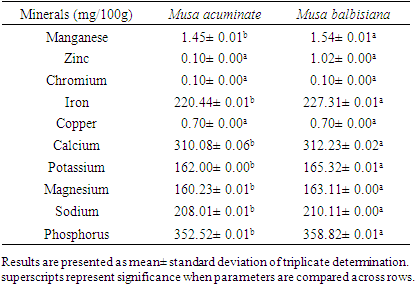
3.3. Mineral Content of Flour Samples
- The mineral contents of the flour samples are shown in table 3. Musa balbisiana has a significantly higher manganese (1.54± 0.01mg/100g) and iron (227.31± 0.01 mg/100g) content than Musa accuminata (1.45± 0.01 mg/100g and 220.44±0.01 mg/100g respectively) while there was no significant difference between the zinc, chromium, and copper content in both flours. The iron content of assayed samples is significantly higher than found in the 47 accessions studies by Fung et al., [31] while the zinc contents are similar ranging from 0.00mg/100g to 1.219mg/100g. Plantains (Musa spp.) are expected to contain 3mg of calcium, 500mg of potassium, 35mg of magnesium, 4 mg of sodium, and 30 mg of phosphorus per 100g [32]. Musa balbisiana contains significantly higher (p<0.05) calcium (312.23± 0.02mg/100g), potassium (165.32± 0.01mg/100g), magnesium (163.11± 0.00 mg/100g), sodium (210.11± 0.00 mg/100g) and phosphorus (358.82± 0.01 mg/100g) content compared to Musa accuminata respectively. This identifies the possibility of variation in the mineral content of plantain varieties.
|
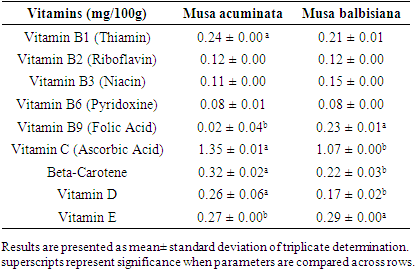
3.4. Vitamin Content of Flour Samples
- Table 4 contains the vitamin content of the flour samples. Plantains are a rich source of vitamins [33]. Thiamin was obtained with 0.24± 0.00mg/100g in Musa accuminata which was higher (P<0.05) than 0.21± 0.01 mg/100g found in Musa balbisiana. The ascorbic acid was significantly different (P<0.05) with 1.07± 0.00 mg/100g and 1.35±0.01 mg/100g recorded in Musa balbisiana and Musa accuminata respectively. Vitamin C is an important nutrient for humans and is readily found in fruits and vegetables [34,35]. The vitamin C content found in the studied plantain flours is remarkably lower compared to results from previous studies [18,36,37]. This implies that plantain flour is not an excellent source of vitamin C. Riboflavin was recorded in Musa accuminata and Musa balbisana were not significantly different with a record of 0.12± 0.00 mg/100g respectively. This result is within the range (0.10 to 2.72 mg/100 g) of riboflavin found in eight plantain cultivars studied by Englberger [38]. It is estimated that 100g of plantain flesh will meet three times the estimated requirement (7mg/day) of Niacin per day [38]. Niacin recorded in Musa accuminata and Musa balbisana was not significantly different with a record of 0.11± 0.00 mg/100g and 0.15± 0.00 mg/100g respectively (P>0.05). Pyridoxine recorded in musa accuminata and Musa balbisana was not significantly different with a record of 0.08± 0.01 mg/100g and (P>0.05) 0.08± 0.00 mg/100g respectively. Folic acid recorded in Musa accuminata and Musa balbisana was not significantly different with a record of 0.02± 0.04 mg/100g and (P>0.05) 0.23± 0.01 mg/100g respectively.
|
4. Conclusions
- This study has shown that the two flour samples had different nutritional contents and functional properties. Flour made from the two varieties of plantain are both nutrient-rich and can be used to ensure food and nutrition security. This will provide the information needed by Dieticians for diet therapy and as well increase its use in the food industry. Efforts should therefore be geared towards ensuring food and nutrition security using plantain as a vehicle in regions where plantain is readily accepted as a staple.
Disclosure
- This section is ONLY for those who requested disclosure. The name of the experts that reviewed your paper, in case they accepted selling disclosure to you, will appear here. Each reviewer is allowed to make their own price for that, since that is a public endorsement of your findings and may be used for varied purposes.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML