-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2022; 12(2): 29-47
doi:10.5923/j.fph.20221202.01
Received: Feb. 15, 2022; Accepted: Mar. 6, 2022; Published: May 24, 2022

A Tribute to Guy Frederic Marrian and Geoffrey Arthur Dering Haslewood. An Overview of the Discovery of Equol and Its Applications in Health and Disease
Wilson D. W.1, Griffiths K. G.2, Takahashi T.3, Tokunaga M.3, Yasukawa Z.3, Nishimura S.3, Horiuchi R.4, Buttar H. S.5, Singh R. B.6, De Meester F.7
1Formerly, School Medicine Pharmacy and Health, Durham University, Durham TS17 6BH, UK; and Centre for Ageing and Dementia Research, Swansea University, SA2 8PP, UK
2Emeritus Professor of Cancer, Cardiff University, Laurel Cottage, Castleton, Gwent CF4 8UR, UK
3Department of Nutrition, Faculty of Nutrition, Kanazawa Gakuin University, 10 Sue, Kanazawa City, Ishikawa Prefecture, Japan
4Department of Food Sciences and Nutrition, Faculty of Human Environmental Sciences, Mukogawa Women’s University, 6-46 Ikebiraki, Nishinomiya City, Hyogo Prefecture, Japan
5Department of Pathology & Laboratory Medicine, Faculty of Medicine, University of Ottawa, Ottawa, ON, K1H 8M5, Canada
6Halberg Hospital and Research Institute, Civil Lines, Moradabad, India
7Masarska 13/127, 31-534 Krakow, Poland
Correspondence to: Takahashi T., Department of Nutrition, Faculty of Nutrition, Kanazawa Gakuin University, 10 Sue, Kanazawa City, Ishikawa Prefecture, Japan.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The early work of Marrian and Haslewood, discoverers of equol, and the much later oestrogenic plant sources of isoflavones, are described, together with clinical applications. Equol in humans may appear in the urine, plasma, saliva, faeces and tissues, and other body fluids, as polar metabolites, but the positions and biological sites of the conjugates must be carefully evaluated before hypotheses on health benefits/risks are generated. Equol enantiomers suggest that phase I metabolism is part of a complex biotransformation of the soy isoflavone daidzein in humans. The potential utility of equol in areas of human medicine is documented; equol is implicated in improving human health in bone, cancer, cardiovascular disease, diabetes, immunology, the gut microbiome, kidneys, liver, reproductive health, skin, thyroid, etc. Two potential dietary innovations are suggested, viz. Equol-enriched functional eggs, and changing diet history and preferences, all governed by strict food security considerations. The limitations on equol research are indicated. This overview calls for a different approach to equol research, e.g. a starting point could be a standard a lifetime dietary system delivered to the dining table, and/or supplements of palatable recreational drinks, nutritional snack bars, capsules: all at affordable prices under a food security umbrella. Regulatory and commercial organisations should work closer together and include equol in their cornucopia of activities on food safety and ‘market claims’.
Keywords: Equol, Isoflavone, Function, Application
Cite this paper: Wilson D. W., Griffiths K. G., Takahashi T., Tokunaga M., Yasukawa Z., Nishimura S., Horiuchi R., Buttar H. S., Singh R. B., De Meester F., A Tribute to Guy Frederic Marrian and Geoffrey Arthur Dering Haslewood. An Overview of the Discovery of Equol and Its Applications in Health and Disease, Food and Public Health, Vol. 12 No. 2, 2022, pp. 29-47. doi: 10.5923/j.fph.20221202.01.
Article Outline
1. Introduction
- It is nearly 90 years since Guy Marrian (Professorial mentor at University of Edinburgh to KG) (alterius non sit qui suus esse potest ([1], p4) first published his work with Haslewood on the discovery and characterisation of equol ([2,3] q.v. (3S)-3-(4-hydroxyphenyl)-3,4-dihydro-2H- chromen-7-ol [4], Figure 1, a non-steroidal hormone [5]): and well over 1200 publications in traditional literature sources alone exist today (16/3/2021) but the puzzle as to why some stallions [3], and humans [6], have irregular urinary levels requires exploration to wit seasonal or chronological variation, dietary factors, metabolism, gut microbiome [7,8], latterly in time possibly human C-Sections [9,10], or even methodological extraction problems [11], or health issues. “So far as can be determined, no dietary factor was the cause of this variation and at present it is impossible to say whether this apparent seasonal fluctuation is fortuitous or not. [3]”: however no apparent useful information was provided on dietary aspects of the urinary horse extracts either supplied by Schoeller of Schering-Kahlbaum A.G., Berlin [2], or Connaught Laboratories, University of Toronto [3]. To answer this question would have required meticulous design, recognition of the Allen[12]-Doisy test [13] for oestrogens involving the assessment of vaginal cornification of oophorectomized mice [14], if applicable from Zondek’s work on urinary oestrogens [15], and e.g. horse breed, type of succulent food, e.g. grassland (It is well known even in the time of Marrian that digestibility in horses may (verb frequently used in this review to express the possibility of being true) vary a little with exercise [16]. Type and composition of grassland herbage varies throughout the year, an abundance in the UK from May to June and decline thereafter with a flush in September and no growth until April. How food was managed [17,18], e.g., herbage, silage, forage and root crops, etc., which vary with season, is important. Dependant on national practice, the most common other succulent foods in the UK were Gramineae (ryegrass), Leguminosae (clovers and vetches), and Brassiceae, whereas those in Canada and Germany will be probably different. To add to the complexity, farm horses require more energy, particularly in winter and require hay and some crops were treated cautiously, for example, whereas ryegrass or Timothy produce good quality hay, leafy clover is more difficult to dry and becomes mouldy causing ‘broken wind’ in horses and abortion in pregnant mares and was probably avoided or managed more carefully [19-21]. Indeed, in the 1930s a narrow tie stall was probably used to house horses with a urinary collection receptacle-type under their rear quarters [22,23] depending on whether mare or stallion, with potentially a range of diets from the feeder or from outside (green grass from grazing fields, hay, forage, lucerne, fresh alfalfa grass, alfalfa pellets or cubes. Different varieties of grains were also given to horses such as barley, oats, lentils, corn, flaxseed, beetroot or pulp, turnip parts or pulp, apple parts or pulp; or broodmares, performance horses, and stallions were given fortified feed containing high quality proteins and amino acids, vitamins, minerals, multiple- grains and forage etc.Was equol acting like an oestrogen or through some other mechanism or was it species dependent [24-28] (Missed opportunity by DWW visiting Prospect, NSW, to study phyto-oestrogens and breast cancer [29]; focussed on protein-ligand interaction [30-33])? Why was this so [34]? Perhaps clues lie in a publication by Ashton [35] (Aberystwyth University Master of DWW). Horses, have a large caecum and colon and have a high capacity for handling roughage, graze on grassland and luscious Leguminosae in summer, possibly favourable to equol, but encounter increased ‘steminess’ of plant material in winter, and need careful management of feed/hay which may not be conducive for high urinary equol production. However, there were two compounds found associated with ketohydroxyoestrin (oxohydroxyoestrin/ theelin [36] / progynon-folliculin [37,38] / oestrone [39,40]: in pregnancy urine) which appeared as contaminants [41-43]; thus, equol remained a latebra factum perhaps pointing to clover [44,45] or other plants as candidates elsewhere in the world. About 40 years later Farnsworth [46] produced a paper showing the prevalence of equol in the ‘plant world’ after research by Bradbury and White [47]. It would have also been potentially confusing for interpretation because clover (Trifolium subterraneum and Trifolium pratense) has other oestrogenic compounds (e.g. biochanin and genistein) [48-52] and white clover [Trifolium repens] has the oestogenic coumestrol [53,54]; or coumestrol per se along with 29 related substances [55]. Now, much more is known about plant isoflavones such their role as phytoalexins [56-58,60] and their antimicrobial response to plant pathogens [59,60] q.v. also maackiain, a pterocarpan, found in red clover and elsewhere, and its dual role as a phytoanticipin [61] and phytoalexin, an interesting area outside the remit of this review. Today our ability to measure equol glucuronide (vide infra) in tissue, e.g., wherein uptake by the brain is low, and as would be expected kidneys are high, and reproductive organs are higher than heart and muscle, etc. [62].Unless otherwise stated in this overview equol generally refers to the S-enantiomer, vide infra, and generally focus has been on human medicine. Equol in humans may appear in the urine [63,64], plasma[65], saliva[66], faeces [67] and tissues as polar metabolites [11,28,68] but the positions and biological sites of the conjugates must be carefully evaluated before hypotheses on health benefits/risks are generated; furthermore the chiral grouping at the ‘3’ position gives rise to enantiomers S(sinister)-(-)equol and R(rectus)-(+) equol or a racemate mixture [69] depending on source.
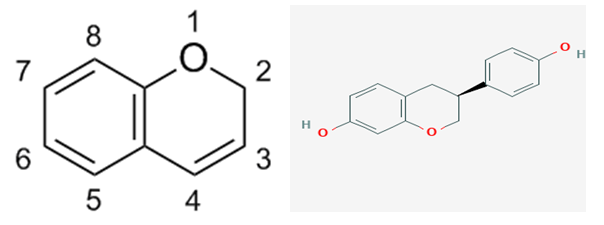 | Figure 1. 2H-chromene structure and ball and stick model of equol, ((3S)-3-(4-hydroxyphenyl)-3, 4-dihydro-2H-chromen-7-ol.) [4] |
2. Equol Conjugates
- Whereas the circulating aglycone in human adult plasma is very low [70], the main conjugated metabolite in plasma is probably (S)-equol-7-glucuronide-4'-sulphate and smaller amounts of 7- and 4'-monoglucuronides and 7- and 4'-monosulphates [71]. The enzymes for glucuronide(s) being UDP-glucuronosyltransferase (UGT) in mammals [28] and is mainly the beta glucuronide [72]; and that for sulphates, aryl sulphatase [73]. Equol is produced by reductases in the microflora of the gut [74-79] from daidzein glycoside and/or formononetin and daidzein is also converted to 5-hydroxy equol [80] e.g. by human Slackia isoflavoniconvertens in both male and female gnotobiotic rats bearing the bacterium and a control. Phyto-oestrogens in the form of isoflavones e.g. diadzin, genistin must be hydrolysed into their aglycones for them to be absorbed and thence functional [81].
3. Equol Metabolism
- Using human liver microsomes, equol was converted mainly 3'-hydroxy- and 6-hydroxy-equol and the aliphatic hydroxylated metabolite 4-hydroxyequol, previously detected in human urine after soy consumption [28], was also identified. These findings suggest that phase I metabolism [82-83] of equol is part of a complex biotransformation of the soy isoflavone daidzein in humans in vivo. S-equol glucuronidation was compared in the liver and intestinal microsomes of mammalia viz. humans, monkeys, dogs, rats, and mice using intrinsic clearance values (CLint) for the sum of 7- and 4'-glucuronidation: and liver>intestine in all species tested, were ‘rats (7.6) > monkeys (5.8) > mice (4.9) > dogs (2.8) > humans (1.0) for liver microsomes, and rats (9.6) > mice (2.8) > dogs (1.3) ≥ monkeys (1.2) > humans (1.0) for intestinal microsomes [28]’ [28]; N.B. humans are standardized to unity. However, regioselective glucuronidation by liver and intestinal microsomes, CLint values were 7-glucuronidation > 4'-glucuronidation for humans, monkeys, dogs, and mice, and 4'-glucuronidation > 7-glucuronidation for rats. Clearly research on equol is dependent on the animal model chosen, ceteris paribus. Metabolomics may be used to signal changes inside and outside of cells e.g. (-)-5-hydroxy-equol may inhibit the proliferation, migration, and invasion of SMMC-7721 cells and inhibit the proliferation of HepG2 cells; which significantly decreased the concentrations of pyruvate, glutamate, and glucose, using 1H nuclear magnetic resonance [84].
4. Chronobiology
- There is a paucity of available data on the temporal impact of equol on regulating human health; and future studies could involve standardised dietary intake of daidzein/equol, in a temporal circadian framework, and identifiable key clinical endpoints such as quantifiable time-qualified ‘endocrine’ [85] or ‘calorific’ [86] markers (oestrogenic isoflavone precursors/metabolites, androgens and oestrogens in plasma), blood pressure measurements, pulse rate, or even metabolomic/lipidomic endpoints such as fatty acids, etc. [87], could be undertaken and diadzein/equol dietary composition correlated with risk of metabolic disorders, e.g. type 2 diabetes or obesity.
5. Potential Utility of Equol in Human Medicine
5.1. Bone Health
- Cautious [88] safety optimism and health benefits [89,90] have been attributed to equol, and the need to recognise EP and NEP status [91-93] when assessing the benefits of isoflavones/ equol in lowering the incidence of osteoporosis in Asian women on a diet rich in soy foods [94] and reducing bone loss in the spine of women with menopausal syndrome [95]. Most, but not all, women show rapid bone loss or osteoporotic disorders after menopause. The rapid bone loss or osteoporosis is clinically important for postmenopausal women, who are relatively at higher risk of bone fractures. Several clinical studies and meta-analysis of randomized controlled trials have suggested that daily ingestion of soy isoflavones at an average dose of 98.2 mg (range: 30.9 – 300 mg/day) for 3-24 months can slow down changes in bone structure in postmenopausal women [96]. Intake of soy isoflavones can also reduce hot flushes and sleep disturbances in the menopausal period. There are numerous mechanisms proposed by which soy isoflavones modulate bone metabolism, however, our knowledge how the soy isoflavones, especially genistein and diadzein, cause reduction in bone turnover rate and increase osteoblastic activity is inadequate and requires further investigations.
5.2. Brain
- Equol exhibited potential blood-brain permeability and anti-neuroinflammatory activity in murine microglial BV2 cells and its cytoprotective effect in a non-contact co-culture model with LPS-BV2-conditioned media and human neuroblastoma SH-SY5Y cells which were evaluated and demonstrated neuroprotection [97] against neurotoxins: probably operating through ERα-mediated pathways [98]. Cognitively normal elderly Japanese, equol-producing status determined six to nine years before an imaging study was undertaken, were significantly inversely associated with the percentage of white matter content [99] (high EP>50% of NEP) but not with amyloid β [100] deposition [101]. Equol may have utility in treating neurocognitive disorders associated with human immunodeficiency virus [102].
5.3. Cancer
5.3.1. Breast (CaBr)
- Equol is expected to have an impact on reducing breast cancer risk (and prostate [103]) by competing for oestradiol for sex hormone binding globulin and the α and β oestrogen receptors [104-106] but there are pros and cons (soy isoflavones; pros-Asians and cons-‘Westerners’ ) possibly arising in part from the EP or NEP status [107-110]. For examples, Dong and Qin [111], using a meta-analysis of prospective studies found an inverse relationship between CaBr incidence for Asians (RR= 0.76 (0.65-0.86) and soy isoflavone diet intake but not ‘Westerners’ (RR=0.97 (0.87-1.06)); and recurrence (RR= 0.84 (0.70-O.99)) but no dose-response was found for total isoflavone intake and CaBr incidence. Trock et al [112] found, using meta-analysis of ‘18 epidemiologic studies (12 case-control and six cohort or nested case-control) published from 1978 through 2004’, that soy exposure and breast cancer risk were inversely somewhat stronger in premenopausal women, in 10 studies for which menstrual status was available (OR = 0.70, 95% CI = 0.58 to 0.85), than in postmenopausal women (OR = 0.77, 95% CI = 0.60 to 0.98) compared to controls. Wu et al. (2002) [113] found that Chinese, Japanese and Filipino females with CaBr and controls were assessed for soy intake in adolescent and adult life; the intake of soy was inverse to risk; similarly high intake was moreso; q.v. [114,115]. The evidence for any role that equol may have from soy on decreasing breast cancer risk is promising but hangs in the balance with ‘may’ expressing hope or possibility.The saga continues into the last decade e.g. equol/isoflavones has been described as having pro-tumour and antitumour effects using cell culture MT5 cells and Tamoxifen [116]; and ‘might’ reduce CaBr development [117]; but equol’s role is ‘unclear’; a list of factors [118] that ‘may’ affect heterogeneities in studies e.g. food source, duration of exposure, oestrogen receptor, and EP status; and perhaps the higher incidence of CaBr in nuns may have a dietary/lifestyle component [119,120] considering that diet may be associated with 10-70% of cancers worldwide [121] but estimates are questionable e.g. [122]. The quotation by Pope may be appropriate [123].
5.3.2. Prostate (CaP)
- Can equol act as an oestrogen and prevent or inhibit prostatic disease development [124]: a recent meta-analysis would suggest not in the case of cancer [125] or from a case-control study nested in the European Prospective Investigation into Cancer and Nutrition study [126] (food sources used by the group are published [127]) and to the contrary for Japanese men but not Europeans? However, equol did inhibit 5α-reductase and 17β-hydroxysteroid dehydrogenase (q.v. phyto-oestrogens [128]) in whole skin fibroblasts and has been found in expressed prostatic fluid from Beijing and possibly implicated in prostatic function [129]. This question has also been addressed some two decades ago by Griffiths et al. [124] mainly on benign prostatic hyperplasia (BPH). Although this review only concerns equol, areas of inter-connecting interest are the epidemiology cancer studies of Doll and Peto [121] and more recently [130], with critics e.g. [122] due to the considerable variation in associative factors; migration studies from widely differing ‘country of origin/host’ CaP (and CaBr) risk e.g. [131]; age-adjusted incidence of Asians vs ‘Westerners’ [132]; oestrogens and the prostate [124]; equol and CaP risk, etc. Clinicians are aware that the prostate is under androgen control by the secretion of testosterone from the testes, mediated through 5α-dihydrotestosterone - first synthesised by Butenandt et al. [133], and localised in the prostate [134-136], and formed by its reductase enzyme located on the nuclear membrane, which binds to the androgen receptor and it is the complex, using preadipose cell culture, that chrysin, flavone and biochanin A, but not equol, which inhibit the aromatase enzyme [137-138]. In work focussed on Tamoxifen (an orphan medicine ([1] (p82), [139]) and Cabr, the complex modulates gene expression. Using androgen-dependant LNCaP cells, equol suppressed cell proliferation and induced apocytosis and the proteosome pathway for inhibition was via the S-phase kinase-associated protein 2 pathway and others [140], and alternative mechanisms [141] gave credence to equol being a potential chemopreventive and therapeutic agent for prostate cancer [142]. Save for the pioneering work of Huggins on bilateral orchidectomy/androgen ablative therapy for advanced cancer ([1], p10), 143,144], the role of oestrogens is more complex both clinically and from a molecular action viewpoint [124,145], including human prostate development processes [146]. Simplistically consider the aging middle-aged man with declining testosterone secretion and increased aromatisation of adrenal androgens and thence increased synthesis of sex hormone binding globulin which in plasma gives rise to higher free oestrogen to free testosterone ratio; thus, increasing the potential oestrogen status of the prostate [124,147]. This system could be modelled by suitable techniques [148,149] duly modified or constructed, though recognition of, even a time-qualified study, and measurement of a physiological variable e.g., testosterone, which if taken as a single plasma sample is not necessarily an indicator of a system pathology [148,150].Thus, reduced clinical benign prostatic hyperplasia in some Asian and Mediterranean men, on isoflavones, may be due to inhibition of growth promoting effects of DHT and oestradiol.
5.3.3. Colon/Rectal Cancer
- It has been hypothesised that dietary isoflavones, e.g,. soy producing equol, may influence colon cancer risk; e.g. plasma equol, measured by high pressure liquid chromatography linked to tandem mass spectrometry, being found to be inversely associated with colorectal cancer risk in a case-control (n=809-809) study nested within the European Prospective Investigation into Cancer and Nutrition study [151]. Measurements (equol by HPLC; microbiota by Matrix Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry) in a small sample size, n=20 subjects with and without (n=20) lesions, of anaerobic microbiota, i.a., Bacteroides fragilis in faecal samples in sporadic colorectal adenomas and urinary equol: the results concluded that there was an inverse association of equol with cancer risk [152]. As may be anticipated, dietary constituents, such as fibre which change the consistency of the diet, may change the activity of the gut microbiota as has been found in women [153].
5.4. Cardiovascular Disease
- EP, through isoflavones or directly, may have a cardioprotective effect for atherosclerosis because of ‘equol’s’ vasorelaxation [154-156] and antioxidant [157] (preventing oxidation of LDL) properties but evidence comes mainly from secondary analysis of primary isoflavone trials and so lack statistical power [158]; a review of 42 articles on coronary heart disease, 14 found that tentatively, equol, possibly providing some oestrogen-like protection [158-160], significantly improved cholesterol and other lipids, inflammation and blood pressure [158]. A narrative review claims that equol is anti-atherogenic and improves arterial stiffness and may be associated favourably with its impact on cognitive decline and coronary heart disease [160]. In one study of isoflavone dietary supplement, lipids were not significantly changed by EP status [161]. Postmenopausal women on isoflavones, overall, did not show a significant reduction in the subclinical progression of atherosclerosis, an inflammatory disease [162], but those randomised within 5 years of the menopause suggest some benefit [162]: but was neither beneficial nor harmful in relation to basal coronary arterial tone and stimulated vasoreactivity and blood flow in patients with coronary heart disease or associated risk factors [163]; or serum lipid reduction in ‘postmenopausal’ women >60y [164]. In subjects with hypertension, isoflavones, compared with gluten, had no effect on arterial function, or 24 h ambulatory blood pressure parameters [165].
5.5. Diabetes
- An increase in pancreatic beta-cell death is associated with problems with insulin secretion and increased risk of type 2 diabetes mellitus. The S-enantiomer of equol reduced alloxan-induced cell death in a dose-dependent manner, acting through a protein kinase A mechanism whereas R-equol had no effects [166] and S-equol may have an anti-diabetic type 2 role. In a matched case-control study of 693 cases (316 women and 377 men) and 698 controls (317 women and 381 men) within the Korean Genome and Epidemiology Group, a design and analysis was stratified according to sex and EP [167] in which isoflavone biomarkers were measured. In women, only a high intake of soya products, indicated by plasma genistein, were inversely associated with risk of type 2 diabetes. No such risk was found in Singaporean Chinese adults in another study [168] and soya protein did not affect glycaemic control in adults. Adipocytes [169], specialised cells of adipose tissue which i.a. store energy as triglycerides, is characterized by sequential changes and expression of regulatory regions of adipose-specific genes and the identification of the transcription factor peroxisome proliferator-activated receptor-gamma (PPAR-gamma). Insulin-sensitizing effects of antidiabetic drugs, are mediated through activation of PPARgamma and it would seem logical to test the effects of daidzein and equol, on adipocyte differentiation and PPARgamma activation using an appropriate cell line such as 3T3-L1 cells [170]; both compounds showed dose-dependent activity suggesting that dietary isoflavones benefits may be attributable to daidzein and equol.
5.6. Immunology
- Soy isoflavones and metabolites do not appear to affect the viability of natural killer (NK) [171] cell signalling and function or healthy donor peripheral blood mononuclear cells (PBMCs) [172], even at high (25 µM) concentrations but pre-treatment of PBMCs with physiologically-relevant concentrations of equol decreases interleukin (IL)-12/IL-18-induced interferon-gamma (IFN-γ) production versus controls (p=0.006). Cellular analyses indicated that equol decreased IL-12/IL-18-induced IFN-γ production by human NK cell subsets but did not consistently alter cytotoxicity. Further research on how dietary soy modulates NK cell functions is needed [173]. In another study [174] both daidzein and equol significantly enhanced, in a dose response manner, the production of interleukin-4 (IL-4), a pro-inflammatory cytokine - closely associated with allergic immune response - in primary CD4+ T cells [175] and EL4 T lymphoma cells, as well as IL-4 gene promoter activity in EL4 cells transiently transfected with IL-4 gene promoter constructs. Both isoflavones increased activator protein-1 DNA binding activities whilst not affecting nuclear factor of activated T cells DNA binding activities. These data suggest that these isoflavones, and others, may increase allergic responses via the enhancement of IL-4 production in T cells.
5.7. Intestine
- Equol is produced by the microflora of the gut [74-80,176-179] from daidzein glycoside and/or formononetin and daidzein is also converted to 5-hydroxy equol [80] e.g. by human Slackia isoflavoniconvertens in both male and female gnotobiotic rats bearing the bacterium and a control. Deep shotgun sequencing [180,181] of the gut microbiome [182] between equol producers (EP) and non-equol producers (NEP) was correlated with serum lipid levels [183) and differed in content and function. Equol in vegetarians (59%) was higher than non-vegetarians (25%) [91]: and in Chinese subjects, on a normal diet (27%) or challenged (60%) with soy-isoflavone supplement, excreted equol [184]: and of the equol producers Adlercreutz equolifaciens 19450T showed higher relative abundance [184] cf. non-producers. ‘Westerners’ have a lower percentage of equol producers e.g. 20-35% who consume soy (soyabean (sp. Glycine max, Fabaceae family)) products [185] or isoflavone supplements [184]; disparity is probably partially due to diet supplement source and possibly antibiotic use [186] or indeed in some cases contamination [187]. Using deep shotgun sequencing and serum lipid profiles equol producers and non-producers were found to differ in metabolic pathways, and at the community and individual level whereupon 32 species were identified with equol production, notably Adlercreutzia equolifaciens (the genus and species are described [188] and Bifidobacterium bifidum [189] had a greater prevalence (EP:- 77.5% vs. 22.5%; NEP:- 72.0% vs. 28.0%) but the stability of equol status has been questioned [6]. Furthermore, it has been reported that there is relative risk of 2.75 (95% CI. 1.00-7.52) for being an equol producer as a child for a maternal EP cf. NEP [190] but faecal equol in children is less developed in those under three (1 in 24) [191] whereas those German children over 6y and in adolescents, urinary equol is highly variable [192]. However there does not appear to be any lasting effect of early isoflavone exposure in early life 4m-7y (n=60) (19% (soya-based infant formula) cf. 5% (cows’ milk) [193]. Equol presumably comes from isoflavone precursors e.g., from vegetables, fruits, etc. as described [192,193] and others [193].Using equol-producing bacteria from a simulator of the Gastrointestinal Microbial Ecosystem (SHIME) [194] may be a boon for NEP subjects; certain dietary isoflavonoids may beneficially quorum sense selective parts of the gut microbiota [195]. Equol and other phyto-oestrogens may act possibly like endocrine disruptors, e.g., bisphenol A, in the brain, and other organs, through activation of oestrogen receptors [196] with possible disruption of: brain programming and resultant neurobehavioral problems [197]; and the reproductive tissue of the developing child - just a cautionary note [198]. The content of the digesta e.g. oligosaccharides, may induce diarrhoea through the accumulation of lactic and succinic acids in the large intestine which inhibits the motility in the large intestine and an increase in viscosity of the digesta may reduce the fermentation rate by decreasing the encounter rate of substrates and bacteria [199].
5.8. Probiotics and Some Food Products
- Probiotic [200] clinical trials are a major area of research in conjunction with isoflavone products. For example, 60 postmenopausal women presenting for symptoms of genitourinary syndrome of the menopause, who were given, i.a,. an isoflavone extract, failed to relieve their vulvovaginal symptoms [201] but did improve isoflavone metabolism as did a prebiotic study [202] ((fructo-oligosaccharides (FOS) and inulin) and sugars (glucose and sucrose)) on enhancing equol production from soymilk isoflavones by Bifidobacterium longum BB536 and Bifidobacterium breveat cc15700 in vitro. β-Glucosidase activity, and therefore assumably, equol production, varied with the soya milk fermentation time; B. breveat cc15700 (max. 36h) and B. longum bb536 (max. 24h) over a period of 48h: but inulin exhibited the highest level of equol production compared to FOS. Research, outside the remit of this overview, is constantly being directed to improving the health of the host using clinical trials of probiotics having a viable strain in the intestine [203]. Reviewing the evolution [204,205] and use of phyto-oestrogens [206,207], in pursuit of equol’s potential ’pot of medicinal gold’ is a major task. In the context of: natural food [208], bovine milk [209] food products [210], genetically modified crops [211]; cultural, economic, environmental [212], and sociological/religious aspects of food and human nutrition in the context of oestrogenicity; together with the immense variety of cuisine activity and interacting other foods, is important. Industrial food processes and product marketing, within regulatory and corporate frameworks (e.g. in Japan [213, 214] and globally [215]) with appropriate quality assurance and tasting panels is beyond the remit of this review.The source of equol depends on the metabolic chain of dietary precursors [216] i.e. isoflavones (3-phenylchroman structure) leading to equol but particular food products, natural or extracts, labelled as phyto-oestrogens, may contain lignans, isoflavonoids or the ubiquitous flavonoids (2-phenylchroman structure) which are high in numerous fruits and vegetables e.g. apigenin (tea) ([124], pp42-43) and kaempferol. Examples of traditional soy products [217] are fermented and non-fermented categories; and some soy isoflavone processing and application has been documented [218-221]. Examples of other supplements include, linseed oil, red clover sprouts, etc.
5.9. Kidneys/Renal
- Limited information from a randomised 3-arm (daily intake of: soy flour; low fat milk + daidzein; low fat milk), 6m trial, of 270 (n=253 finished) prehypertensive postmenopausal Chinese EP women showed no substantial change/difference of treatment on most renal parameters except those with lowered renal function where there was modest improvement on soy flour [222]; and renal function was not improved in patients with diabetic nephropathy according to phyto-oestrogen levels, including equol, in serum and urine suggesting a lack of major protection effect on the development of diabetic renal and cardiovascular complications [223]. The potential vagaries of a 24h urine sample collection may be important [224], including chronobiological considerations.
5.10. Liver
- Early research indicated that equol was negatively associated with percentage of free oestradiol in plasma thus possibly implicating equol in oestrogen metabolism [225]. Adopting the concept of Griffiths et al. [147], dietary oestrogens may inhibit the DHT and oestradiol growth promotion of the stromal tissue of the prostate and, also the inhibition of peripheral aromatization of adrenal androgens which may reduce clinical benign prostatic hyperplasia (BPH). The liver will moderate its sex hormone binding globulin secretion and consequently its relative binding of testosterone and oestradiol in plasma i.e., androgen-oestrogen balance, and reduce the risk of BPH ([147] - pp88-92). The glycosidation and sulphation of equol in the liver of humans [91] is discussed in the ‘Equol metabolism’ sction of this review. In a human hepatocellular carcinoma SMMC-7721 cell line (±)-equol, R-(+)-equol, and S-(-)-equol inhibited proliferation in a dose-dependent manner with significant cell cycle arrest in the S-phase. It also caused endoplasmic reticulum stress-mediated apoptosis through intrinsic and extrinsic pathways by activating caspase-8 and caspase-12 [226,227] and by upregulating Chop and Bip [228,229]. Mitochondrion-mediated apoptosis was caused by upregulation of Bax and downregulation of Bcl-2, and activation of caspase-9, caspase-3, and cleaved poly (ADP-ribose) polymerase [230], [228,231]. From a cancer perspective a nested case-control study of plasma isoflavones and risk of primary liver cancer in Japanese women and men with hepatitis virus infection was undertaken and equol, along with other isoflavones, was not statistically associated with this cancer [232]. Interestingly, metabolomics has been used to study the effect of equol on cancer cell lines, vide supra [84].
5.11. Reproductive Tissues and Health
- In a major symposium in 2010 on isoflavone products and peri- and post-menopausal women’s health, conclusions were mixed [233] as sometimes is ‘the literature’. Treatment of postmenopausal osteopenic women with a well-tolerated red clover isoflavone extract (RCE) attenuated bone mineral density loss [234] whereas in a cross-over study of total isoflavones in postmenopausal women of calcium retention, remained unchanged in EP and NEP subjects [235], similarly, for soy supplement rich in daidzein did not change bone mineral density though there may be differences in circulating free oestradiol and oestrone [235]. In elderly Chilean women with femoral osteoporosis no improvement in bone mineral density was observed with a special supplement including isoflavones [236]; in yet another related study, equol had no apparent effect on bone mineral density [237]. Apparently healthy white premenopausal women on a long-term (1y) diet enriched with isoflavones did not prevent postmenopausal bone loss nor affect bone turnover [238]. In a study of isoflavones and postmenopausal women, markers of inflammation are not changed significantly [239]. However, in postmenopausal women, S-equol, 10 mg/day, appears comparable to, or even better than, soy isoflavones at reducing hot flush rates; and is more effective for relieving muscle and joint pain [240]. Premenstrual syndrome (PMS) may be a risk factor for NEP Japanese women: a study of EPs in controls (n=98) and PMS patients (n=46) was just significant (P=0.04) [241] with an OR=2.3 (95% CI 1.02-5.7). In another study of female athletes NEPs were associated with poor athletic performance [242]. Studies pertaining to peri- and post-menopausal women on equol still, in 2021, need to be taken cautiously [243].
5.12. Skin
- Topical treatment of ageing, or oestrogenic-deficient, women’s skin, often associated with the menopause, with equol, is a promising area of research [244-246], and is patented [247], because such deficiency leads to ‘loss of collagen, elastin, fibroblast function, vascularity, and increased matrix metalloproteinase(s) enzymatic activities, resulting in cellular and extracellular degradation that leads to dryness, wrinkles, atrophy, impaired wound healing/barrier function, decreased antioxidant capacity, decreased attractiveness and psychological health, and increased perception of aging’ [248]. Rather than use systemic oestrogen to reverse the ageing process, acting through the ER-β [106], oral equol dietary supplement targets these receptor molecules found in keratinocytes/fibroblasts which uses selective oestrogen receptor modulators (SERMs) to combat ageing [249,250]. Nutricosmetic potential of equol-producing Pueraria lobata (source of isoflavones) extract fermented with L. paracasei JS1 (equol producing bacteria) on the skin to reverse skin ageing is possible [251]. Equol may even protect the skin from UV radiation damage [252].
5.13. Thyroid
- Relatively little is known about the effects of equol on the thyroid gland. One study on male gavaged rats, receiving equol with flutamide control, demonstrated that equol did not affect the anti-androgenic pathway of the hypothalamic-pituitary-thyroid axis [253], assessed by immunoassay of thyroid hormones and TaqMan® which is a real-time reverse transcription polymerase chain reaction [254]. Although flutamide [255] significantly reduced relative prostate weight, equol displayed no such effect but equol did cause down-regulation of hypothalamic thyrotropin-releasing hormone mRNA expression.
6. Dietary Innovations
6.1. Equol-Enriched Functional Eggs
- Soy isoflavones are known to efficiently transfer from chicken feed to egg yolk making the latter an even better source of nutrients with potential benefits to human health, including phytoestrogens. Isoflavones (daidzein, glycitein, genistein) appear to be turned into aglycones and microbial metabolites all along the gastrointestinal tract of the bird. In particular, the soy isoflavone-glycoside daidzin is efficiently metabolized and transferred - as equol conjugates - all the way from the intestinal tract to the yolk of the egg (mostly in the granule fraction) [256] by way of blood lipoproteins (mainly HDL). This opens the door to the design of functional eggs enriched/rich in active forms of phytoestrogens which may ideally complement human nutrition, especially in women and infants.
6.2. Equol: Changing Diet History and Preferences
- It is assumed from the available evidence cited herein, that equol from dietary sources may bring health benefit, particularly taken in childhood [257], to the prevention or arrest of non-communicative diseases. This is a major cause of death in high-income countries [258] wherein diet is a major risk factor [259], then the impediments to dietary adoption must be overcome, such as dietary preferences: of adults that may begin in childhood, even kindergarten [260], and will be culture and country specific; personal likes and dislikes [261]; provision and nature of school meals [262]; needing appropriate design and analysis e.g. [263]; family income; urban/rural location for access to nutritious food; soy food industry products (a major area beyond the scope of this review, e.g. [264]); etc. [265].
7. Discussion
- This is an atypical discussion that tries to elicit the state of balance between what research on equol has promised and achieved, and what it has failed to deliver thus far; and suggestions on what needs to be done to realize its potential or otherwise. It is likened to a multidimensional jigsaw puzzle with missing pieces, should they exist, to complete a picture or non ens. The aglycone equol, and enantiomers, was one of a plethora of discoveries related to steroid hormones in the 1930s that seemed to have oestrogen-like potential, opening the door to a possible myriad of uses in human medicine, particularly as it is found in many body fluids. On the plus side, equol has been implicated in improving health in bone, cancer, cardiovascular disease, diabetes, immunology, the gut microbiome, kidneys, liver, reproductive health, skin, thyroid, etc. The armamentarium of research tools concerned with equol and dietary studies included epidemiology (coping with variability), clinical trials (criteria for subject selection), meta-analysis (criteria for study selection), in silico molecular docking [266,267] (choice of expressed proteins in disease), metabolomics (integration with genomics, transcriptomics, proteomics), PCR and TaqMan (synthesis of probes), cell lines (biological integration of individuals), food technology (reliable health information), etc.,: an example of disadvantages is shown in parenthesis for each tool. Subjects have a lifetime from gastrulation to death and are influenced by biological, social, chronological and cosmic factors that exist on Earth, as does the space in which they take place [268,269], wherein diet composition, such as isoflavones, is an important part of existence but is rarely documented in sufficient detail to design experiments to obviate confounding factors. It would seem that a different dietary strategy is needed, involving functional foods such as isoflavones (equol or precursors), and other none-nutrients of plant origin, to improve human health. A short excerpt from a primary publication from one of our co-authors [270] is absolutely relevant.
 A starting point could be an advisory marketable multifunctional lifetime dietary system or supplement options, commensurate with the inbox, delivered to the dining table whencesoever that may be, and/or supplements of palatable recreational drinks, nutritional snack bars, capsules, all at affordable prices. Consumption could be monitored and stored using an ‘App’ and data used in research studies; benefits could arise from health insurance premiums and entrepreneurial marketing, most of all from a happier family life and a more contented mankind: a gargantuan task but substantially achievable in this century in which isoflavones/equol may have a role. Finally, Natural Selection in Homo sapiens evolutionary development, during the last 200,000 or so years, within different habitats, cultures, religious and social practices, may mean there is not a ‘correct diet’ for all mankind [271] as described, for example, by dichotomies in cancer risk in EP and NEP, herein. Many authors have made significant advances on the role of equol in human health but more work is needed to ensure that equol attains its proper place, whencesoever it is truly realised.
A starting point could be an advisory marketable multifunctional lifetime dietary system or supplement options, commensurate with the inbox, delivered to the dining table whencesoever that may be, and/or supplements of palatable recreational drinks, nutritional snack bars, capsules, all at affordable prices. Consumption could be monitored and stored using an ‘App’ and data used in research studies; benefits could arise from health insurance premiums and entrepreneurial marketing, most of all from a happier family life and a more contented mankind: a gargantuan task but substantially achievable in this century in which isoflavones/equol may have a role. Finally, Natural Selection in Homo sapiens evolutionary development, during the last 200,000 or so years, within different habitats, cultures, religious and social practices, may mean there is not a ‘correct diet’ for all mankind [271] as described, for example, by dichotomies in cancer risk in EP and NEP, herein. Many authors have made significant advances on the role of equol in human health but more work is needed to ensure that equol attains its proper place, whencesoever it is truly realised. 8. Summary
- From the early discovery of equol by Marrian and Haslewood, a colossal amount of work has been carried out which suggests that there is, as yet, not fully discovered, an equol network which may regulate human health. New research vistas need to be sought, and regulatory and commercial organisations should work closer together and include equol in their cornucopia of activities on food safety and ‘market claims’.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML