-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2021; 11(2): 44-52
doi:10.5923/j.fph.20211102.02
Received: Oct. 22, 2021; Accepted: Nov. 13, 2021; Published: Dec. 15, 2021

Biological Evaluation of Cereals and Legumes Weaning Blends for Infant Weaning Food
Funmilayo G. Adebiyi1, Kofoworola I. Adediran2, Omobolanle A. Olaoye3, Aderonke O. Mosuro4, Oluwatobi A. Olaomi5, Olugbenga A. Ogunwole1
1Agricultural Biochemistry & Nutrition Unit, Department of Animal Science, University of Ibadan, Nigeria
2Institute of Child Health, College of Medicine, University of Ibadan, Nigeria
3Animal Husbandry Services, Ministry of Agriculture and Rural Development, Oyo State Secretariat, Agodi, Ibadan, Nigeria
4Human Nutrition and Dietetics Department, Faculty of Public Health, Lead City University, Ibadan, Nigeria
5University College Hospital, University of Ibadan, Ibadan, Nigeria
Correspondence to: Olugbenga A. Ogunwole, Agricultural Biochemistry & Nutrition Unit, Department of Animal Science, University of Ibadan, Nigeria.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The production of low cost and high-quality nutritional foods for infants is a major challenge in developing countries which often contribute to the high prevalence of malnutrition with its attendant risks. Consequently, the biological assessment of locally available cereal and legume blends as weaning foods for the feeding of infants was investigated in this study. In a 10-day trial, weanling male Wistar rats (n=36) were randomly allotted to nine treatments, each in quadruplicate. Diets 1 to 7 consisted of different cereal multimixes; Diet 1 (millet 40: malted millet 20: soyabean 30:sucrose 10), Diet 2 (corn 40: malted corn 20: soyabean 30: sucrose 10), Diet 3 (millet 20: malted millet 10: corn 20: malted corn 10: soyabean 25: groundnut 5: sugar 10), Diet 4 (millet 20: malted millet 10: corn 20: malted corn 10: soyabean 25: crayfish 5: sugar 10), Diet 5 (sorghum 70: malted sorghum 10: soyabean 20), Diet 6 (corn 70: soyabean 30), Diet 7 (sorghum 60: malted sorghum 10: soyabean 15: cowpea 15). Diet 8 was the control, casein-based diet while Diet 9 was the nitrogen-free diet. All Diets, except Diet 9, were isonitrogenous, formulated to have approximately 10% crude protein. Feed and protein intake of rats on all test diets were similar (p>0.05). Feed conversion ratio were significantly higher (p<0.05) for diets 1(7.52), 2(7.47), 5 (8.01) and 6(7.96) than 3(6.42), 4(6.40), 7(6.91 and 8(6.37). The protein efficiency ratio (PER), net protein retention (NPR), protein retention efficiency (PRE) and net protein utilisation (NPU) varied significantly (p<0.05) with diets. Biological values were similar (p>0.05) for diets 4 and 8 but significantly higher (p<0.05) than 3 and 7 as well as 1, 2, and 6, while diet 5 was least. Therefore, Diet 4 -the blend of cereals, legumes, crayfish and sucrose had the best protein quality and could therefore be suitably deployed as infants weaning food.
Keywords: Biological values, Weaning Wistar rats, Feed conversion ratio, Infants weaning food
Cite this paper: Funmilayo G. Adebiyi, Kofoworola I. Adediran, Omobolanle A. Olaoye, Aderonke O. Mosuro, Oluwatobi A. Olaomi, Olugbenga A. Ogunwole, Biological Evaluation of Cereals and Legumes Weaning Blends for Infant Weaning Food, Food and Public Health, Vol. 11 No. 2, 2021, pp. 44-52. doi: 10.5923/j.fph.20211102.02.
Article Outline
1. Introduction
- Proteins are the major functional and structural components of all body cells [1]. Protein plays an essential role in the development of muscles, skin, and production of enzymes and hormones in the body [2]. The quality of protein given to children in their formative years is critical to their mental and general development. Inadequate intake of protein by infants could lead to kwashiorkor in children. An earlier study [3] revealed that a reduced protein intake or low-quality protein intake plays a critical role in the metabolic health and longevity of infants.Malnutrition and undernutrition have been a long-term concern of governments in developing and underdeveloped countries. This is due to their effects on the physical, economic, social, and political development of a country [4,5]. Unlike in developed countries, where true protein deficiency may be rare, the state of nutrition in Nigeria is generally characterized by inadequate protein supplies, especially during the formative years of infancy and childhood [2]. The gross insufficient protein supply is not only ascribed to inadequate production of large quantities of edible proteins but also due to a dearth of high-quality proteins [6]. UNICEF [7], reported that Nigeria has the second highest burden of stunted children in the world. Most of these children, are under the age of five and were observed to be suffering from severe acute malnutrition. High-quality protein intake is therefore critical for efficient growth and development of children. Nutritional deficiencies, usually have far-reaching effects on the general well-being of children. Children with severe acute malnutrition have a reduced ability to respond to stressors (including infections) and are at risk of death [8]. Malnutrition may also result in mental retardation of the surviving children which may be carried over to future generations [9].A recent survey by the Nigeria National Bureau of Statistics on the nutrition status of under five children in 37 states of Nigeria showed that acute malnutrition remains at alert levels. Chronic malnutrition as characterised by stunting was at serious or high levels according to the classification of WHO/UNICEF [10]. The prevalence of stunting was 32% and has remained the largest burden of malnutrition with stagnant rates of above 30 per cent since 2014. Also, many states in the northwest and northeast of Nigeria recorded prevalence levels above 40% of the WHO critical levels [10].The greatest single factor which probably contributes to the survival of most African children has been the widespread acceptance of breastfeeding as a normal way of life by African women [11,12]. However, at six months of age, breastmilk alone would be inadequate as the sole source of nutrients for the infants. Hence, complementary foods will be introduced while breastfeeding continues through the second year of the child's life in many traditional African societies [1,13].Traditional complementary foods in most developing countries, including Nigeria, have been described as being inadequate for infants in terms of nutritional quality. These traditional weaning or complementary foods are low in protein content and devoid of vital essential nutrients for normal child growth and development [14-16]. For instance, the corn gruel (ogi), which is the traditional weaning food of infants in many parts of West African countries, has been reported to have low protein content, which cannot meet the daily nutritional requirements of infants [17-20].The emergence of the COVID-19 pandemic seems to also take a toll on the nutrition and general well-being of Nigerian children [21]. The maintenance of a high level of nutritional health requires a consistent availability of low cost, wholesome and nourishing foods or diets that will provide essential nutrients in quantities sufficient to meet infant needs. In the face of the economic recession occasioned by COVID-19, there is a need for more concerted efforts to provide nutritious, balanced, and acceptable foods to most African children. This can be achieved by the use of locally available grains and legumes blends rather than reliance on commercial foods. Though most commercial weaning foods have been formulated to meet the nutrient requirements of infants, they are, however, relatively very expensive and beyond the reach of low-income families in developing countries [1,22,23]. Therefore, developing nutrient-dense, safe, affordable and accessible complementary food from locally produced ingredients should be of ultimate concern in addressing the problem of malnutrition [24]. Previous studies have been carried out to evaluate the functional, nutritional, sensory qualities and proximate composition of some combinations of tubers, cereals, fruits, and legumes [15-17,20]. Report on the proximate composition of weaning food processed from cooking banana fortified with cowpea and peanuts [25], showed that the blend could serve as a supplementary food and a transition meal from breast milk to solid family diets in Nigeria. Also, popped indigenous cereals and legumes were shown to be suitable supplementary food for feeding rural mothers and children in India [26]. The physicochemical quality of nine instant weaning foods formulated from cereals, legumes, tubers, vegetables, and crayfish have also been evaluated [27]. This study indicated that the multimix diet had the desired characteristics of an infant weaning food and could be used to alleviate the protein-energy malnutrition in Cameroon. More information is however needed on the biological value of different combinations of legumes and malted grains as potential food for infants. Consequently, this study was aimed at assessing the biological values as well as digestibility of various blends of malted cereals and legumes as a potential complementary food for infants.
2. Materials and Methods
2.1. Preparation of Test Ingredients
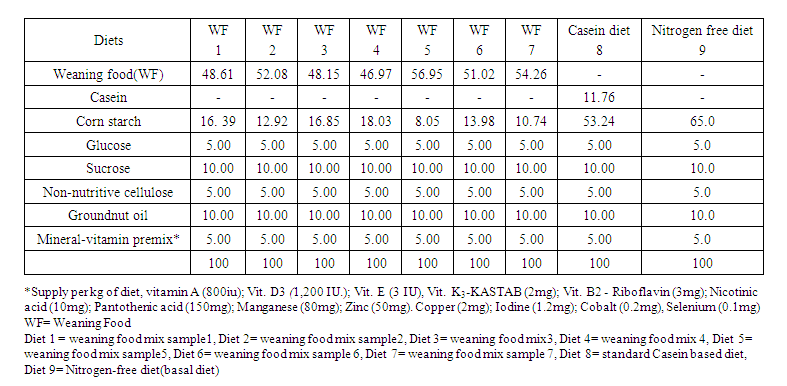
- The cereals and legumes used in this study were purchased from Oshodi Market in Lagos, Nigeria. The grains were sorted to remove dirt and bad grains. Portions of the grains (i.e corn, millet, sorghum, and soyabean) were roasted in a pan mounted on a wooden charcoal heat source for ten minutes until uniform brownish taint was achieved, while the other portions of sorghum, millet and corn were malted. The malting was done by soaking a portion of the corn, millet, and sorghum in cold water for five days. The water was thereafter drained, air-dried for 24 hours and subsequently, oven dried at 80°C until constant weights were attained. The properly oven-dried grains were milled to pass through 0.2mm sieve and incorporated into the test weaning diet as shown in Table 1.
|
2.2. Composition of Weaning Food
- The weaning mixes contained blends of different cereals and legumes. The constituents of each weaning mix are shown in Table 1. The selection of the constituents was based on the protein content of each ingredient and any combination that could provide the acceptable WHO recommended nutrient intake for infants.
2.3. Management of Experimental Animals
- The feeding phase of this experiment was carried out in the rat house of the Department of Animal Science, University of Ibadan, Ibadan, Nigeria. Weanling male Wistar rats (n=36) were procured from the Animal House, Department of Physiology, College of Medicine, University of Ibadan, Ibadan, Nigeria. The rats weighing 25.86±2.02g were housed in individual thoroughly cleaned and disinfected stainless-steel metabolic cages designed with facilities for a separate collection of faeces and urine. The stainless-steel metabolic cubicles (Multiple Compartment Rack Feature One Cage Model 1000, England) with 0.35 x 0.22 x 1.35 m dimension. The cages were set up in two layers of six cubicles and four cage sets were housed in a 41.25 m2 room. The cages were kept in a clean and well-ventilated room. Rats were allowed to acclimatise for five days on diets prior to data collection on rats for the next five days. The rats were randomly allocated to nine different treatments, each replicated four times. One group was offered a casein diet (control), another group received a nitrogen-free diet (Basal diet), while other groups were fed test protein diet as shown in Table 2. Feed and water were given ad-libitum.
2.4. Composition of Test Diets
- The test diets contained blends of different cereals and legumes (i.e the weaning mixes) which were incorporated into a basal diet, i.e., a nitrogen-free diet (NFD) at the expense of corn starch, such that the diet provided 10% crude protein in the final diets and the unmodified basal diet served as the nitrogen-free diet (Table 2).
 | Table 2. Dietary Layout of the Experimental Diets |
2.5. Parameters Measured
- Daily ort was recorded to ascertain the amount of feed taken daily by each rat. The final weight of each rat was also recorded at the end of the experimental period, which lasted for 10 days. Feed conversion ratio, protein efficiency ratio, Net protein retention, protein retention efficiency, net protein utilization and biological value were calculated using the following formulae as described by Pellett, and Young [28], respectively in equations 1, 2, 3, 4 5, 8 and 9 below. Faecal and urinary collections were made on the last five days and the daily weights of faeces collected from each rat were recorded. The faecal samples were oven-dried to constant weight at 85°C. The weights were recorded to determine the moisture content of the faeces. The dried samples were ground mechanically with a laboratory mortar and pestle and stored in the airtight Kilner jars until required for analysis. The true digestibility and apparent digestibility of crude protein were calculated using the formulae in equations 6 and 7 as documented by Bhutta and Sadiq [29].
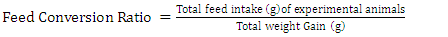 | (1) |
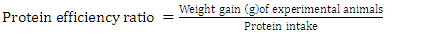 | (2) |
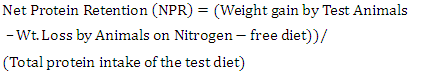 | (3) |
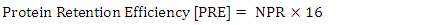 | (4) |
 | (5) |
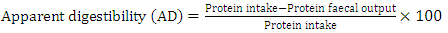 | (6) |
 | (7) |
2.6. Preparation of Rat Carcasses for Analysis
- This experiment was terminated at the end of 10 days. The rats were weighed and sacrificed using chloroform inhalation. Incisions were made on the head and from the lower jaw to the abdomen to enhance uniform drying. The carcasses were then dried in an oven at 85°C to a constant weight, milled and stored until further analysis. Carcass nitrogen was determined as described by [30] and as modified by [31]. Protein was calculated as N × 6.25. The carcass nitrogen and nitrogen intake for the ten-day feeding period were used to compute protein quality parameters and biological value as shown in equations 7, 8, and 9.
 | (8) |
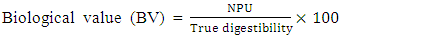 | (9) |
2.7. Chemical Analysis
- The proximate analyses of the weaning mixes, experimental diets, carcass nitrogen, faeces and urinary nitrogen were carried out according to the standard methods of AOAC [32]. The analysis was carried out in triplicates.
2.8. Statistical Analysis
- The data were analysed using the completely randomized design model (SPSS Version 25). The Duncan multiple range test was used to separate the means. Significant variation was accepted at p<0.05.
3. Results
- The proximate analysis of the experimental diet is shown in Table 3. The results of the proximate composition of the various samples of weaning food are shown in Table 4. The crude protein content ranged from 17.41% for weaning food five to 21.31% for sample four. The crude fibre ranged between 2.69% (weaning food 6) and 3.55% (weaning food 3). The ether extract ranged from 4.22% for weaning food two to 5.24% for weaning food three. The ash also ranged from 2.98% for sample five to 4.14% for sample four. While the NFE ranged from 66.91% for sample four to 72.06% for sample five.
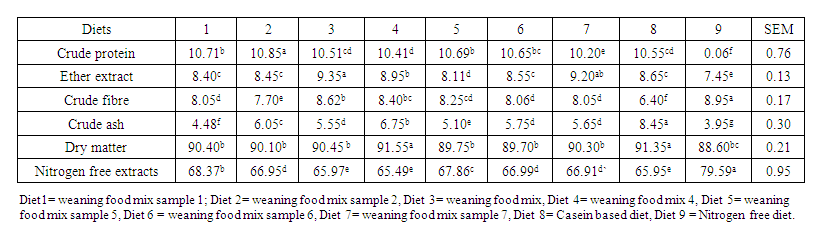 | Table 3. Proximate analysis of experimental diets (g/100g DM) |
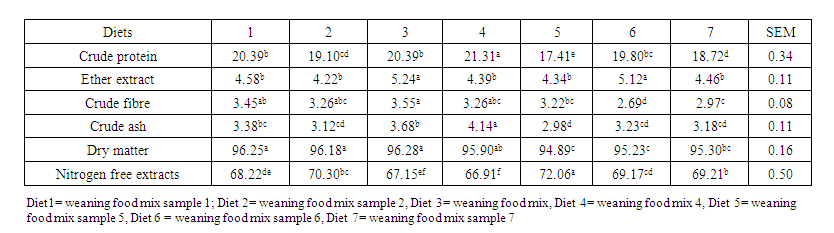 | Table 4. Proximate composition of weaning foods [g/100g] |
3.1. Performance Characteristics
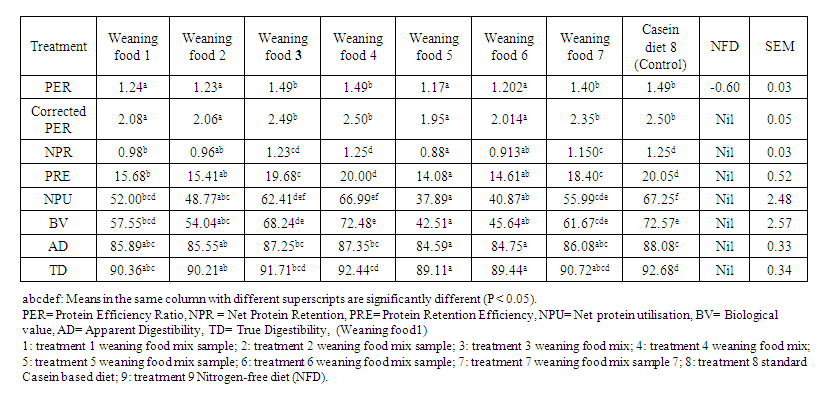
- The relative effects of the various weaning foods on the performance of albino rats are shown in table 6. The feed intake was not significantly different (p>0.05) for any of the treatments. Protein intake was also not significantly (P>0.05) affected. However, the different weaning mixes had a significant effect on the weight gain of the experimental rats. Rats fed the control treatment ration (casein diet) had the highest body weight gain of 8.09g, which was similar to the weights of rats fed diets 4 and 3. Rats on dietary treatments 5 and 6 recorded the least weight gain with values of 5.34g and 5.52g respectively. These values were significantly lower than the weight gain of rats fed diets 3, 4 and 8. Likewise, the rats fed casein meal (control) had the lowest feed conversion value of 6.37, which was significant (P<0.05) when compared with rats fed diets 1,2,5 and 6.Table 6 shows the protein quality and digestibility of weaning mixes made from various blends of cereals and legumes fed to albino rats.
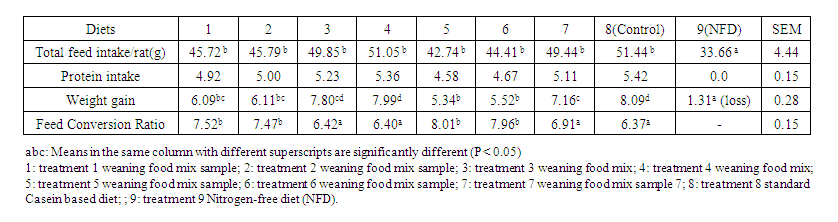 | Table 5. Performance Indices of Weanling Wistar Rats Fed Weaning Mixes |
 | Table 6. Biological Quality of the Weaning Food Mixes fed to Weanling Wistar Rats |
4. Discussion
- The protein composition of the weaning foods showed a greater improvement when compared with the traditional weaning or complementary foods which were just made up of only corn or millet. The crude protein content ranged between 17.41% and 21.31%, which is within the WHO's recommended dietary allowance [1], which recommended a protein intake of 13g for 0-5-month-old infants and 20g-24g for four years old children. The Ether Extract, crude fibre, and ash values of the weaning food also fall within the recommended dietary allowance. This indicates that the diets are good quality food for infants.The value of the total feed intake and protein intake which showed no significant difference (at p<0.05) when the test diets were fed to albino rats indicate a fairly similar consumption of all diets regardless of the crude fibre content of each diet. The results of the weight gain of the experimental animals were partly influenced by feed intake as well as the essential amino acid pattern of the dietary protein fed to the animals. The average weight gain of rats fed the casein diet was comparable to the weight gained by rats fed on diets 3,4 and 7. This could be an indication that weaning mix 3,4 and 7 could provide the quality protein needed to compete favourably with the casein diet in synthesising body tissues for growth. This result is in agreement with a previous study that recorded that a mixture of maize, soybean and nuts supported positive growth of rats [15]. The lowest weight gain observed in rats fed diet 5 (80% sorghum and 20% soyabean) is similar to an observation of a study that recorded a reduction in feed intake and weight gain in rats fed a sorghum-based diet when compared with the rats fed control diets [33]. The lower weight gain could also be attributed to the low protein intake of rats fed the sorghum-soybean diet as well as the tannin content of sorghum. Rats fed the casein diet recorded the lowest FCR, though the value was comparable to the FCR of rats fed diets 3,4 and 7. The comparable values of FCR in rats fed diets 3,4 and 7 with casein diet may probably be because each of these diets consisted of at least 3 sources of protein which complemented each other in terms of essential amino acid availability, indicating a better utilization of feed by experimental rats.Significant differences were also observed in all the protein quality parameters, i.e. PER, C-PER, NPR, PRE, NPU, and BV. The values of protein quality parameters obtained in this study indicated that protein was efficiently utilized by rats fed casein diet, diets 3, 4 and 7. In addition, the PER and C-PER values obtained for these diets were significantly (p<0.05) higher than rats fed on diets 1,2,5 and 6. This may be due to the presence of complete essential amino acids in these rations compared to others. The highest numerical value of PER after casein diet was observed in rats fed diet 4, which consisted of plant and animal sources of protein that perfectly complemented each other in terms of amino acid balance. This is in line with the findings of Mahgoub [34], who observed a similar PER in rats fed cereals-skimmed milk mix and a casein/cerelac diet. The high value of PER in diets 3 and 7, which were favourably compared with that of the control diet, also agrees with an earlier study that recorded higher values of PER in rats fed a mixture of maize-soybean-groundnut-moringa diet [35]. The same trend of PER was observed in the corrected PER values. The corrected PER values of all the diets assessed (except for diets 6 and 5), were in the acceptable range recommended by the United Nation protein advisory group reported in PAG guidelines number 8, [36] in which the minimum acceptable C-PER is 2.1.The nutritional value of the protein content of the various blends of cereals and legumes in terms of NPR and PRE showed no similarity among treatments. The lowest NPR and PRE values were observed in rats fed diet 5 (sorghum-soybean mix), indicating a lower protein retention capacity of the weaning foods by the experimental animals. The low protein content of diet 5, when compared to other diets, might have contributed to the low NPR and PRE recorded in this study. The highest values of NPR and PRE were observed in rats fed the casein diet and diet 4 (Millet-Corn-Soyabean-Crayfish diet). The high net protein retention (NPR) value of diet 4 when compared to the remaining weaning diets, is probably due to the inclusion of soybeans and crayfish meal; which could be a better complementary amino acid. This observation is consistent with an earlier study that revealed that fortification of cereals with protein from animal and or vegetable sources, particularly cashew nuts, soybeans, and or fish, usually improves their nutritional quality [15]. Ene-Obong and Obizoba [37] also confirmed crayfish protein as a better supplement to legume/cereal or legume starchy stable mixtures than leguminous oilseed. In the same vein, recent findings also suggest that the addition of crayfish to locally made infant food (ogi (pap)) could improve the quality of the protein [17]. The values of BV and NPU were significantly affected by the proportion and quality of protein in the weaning foods. The NPU values of rats fed on diets 4 and 3 compared favourably with the rats fed casein diet (control), though significantly higher than other treatments. This is an indication that the feeds consumed were well utilised by the experimental animals. This was further confirmed by the NPU values being higher than the 60% value recommended by Protein Advisory Group [36] for weaning foods. Furthermore, the similarity in biological value for rats fed the control diet and diets 3,4 and 7, seems to suggest that the blend of cereals, legumes, and crayfish mixes provided a good combination of essential amino acids which were available for body tissue synthesis in the rats under study. This is also in agreement with a previous study that recorded that the nutritional quality of most plant-based food materials improved, particularly when combine [16]. Our result also corroborates the observations in a systemic review, wherein most of the assessed Nigerian plant-based staple foods were of low protein quality and predominantly lacked the amino acid lysine [6]. The addition of animal-source foods can bridge the protein quality gap created by the predominance of plant-based foods in the Nigerian diet [6]. The similarity in BV and NPU for rats fed the control diet and diets 3 and 4 tended to suggest improvement in protein quality as well as the utilisation of these diets when fed to the experimental animals. The high values of BV and NPU of rats fed diets 3 and 4 could also be an indication that combinations of cereals, animal protein and legumes could improve the protein quality of food, This also corroborates with the findings of Makanjuola [38] who reported improvement in the protein quality of maize meal when combined with locust bean seeds. The low BV and NPU values of rats fed diet 5 (sorghum-soybean) is a clear indication of the inferiority of the protein quality of this diet when compared to others. The high percentage of sorghum in diet 5 might have contributed to low values of measurement of protein quality observed in this study.The values of AD and TD of rats fed diets containing weaning mixes were lower than that of casein diets. This may be attributed to the incorporation of legumes into weaning food. Legumes have been reported to have a lower digestibility than casein diet [39]. The lowest values for AD and TD observed in diet 5, which had a high percentage of sorghum (80%) seems to suggest that there was no mutual supplementation effect between sorghum and soybean protein at that level of inclusion. Studies have shown that some antinutritional factors embedded in some legumes and cereals may adversely affect protein digestibility [39-40]. Drulyte and Orlien, [40] reported that the anti-nutritional proteins like trypsin inhibitors and lectins and the anti-nutritional chemicals like tannins, phytates, and polyphenols in legumes could have a significant effect on the digestibility of different types of legume protein. Also, the differences in the protein digestibility of the various weaning foods may arise from inherent differences in the nature of food protein as well as the percentage composition of various combinations of the cereals and legumes in each food and the presence of antinutritional factors occurring naturally or formed during processing [41-42].However, rats fed diets 3, 4 and 7 showed comparable apparent and true digestibility values with the casein diet. The improved digestibility of the cereal proteins in these diets might have been due to a greater mutual supplementation effect of more than two sources of protein in the diet. The high values for AD and TD in diet 4 could be due to the presence of animal protein as revealed in an earlier study that recorded a marked increase in digestibility when legumes were cooked with other proteins, particularly animal products [43].
5. Conclusions
- The weaning mixes were analytically adequate in nutrient composition for infant feeding. However, the biological values of the diets and performance characteristics of the experimental animals were significantly different. The standard casein diet and the diets containing millet + malted millet + corn + malted corn + soybeans + groundnut + sugar (20-10-20-10-25-5-10) (i.e diet 3) and millet + malted millet + corn + malted corn + soybeans + crayfish + sugar (20-10-20-10-25-5-10) (i.e diet 4) gave the best nutritional and biological quality. This study revealed that weaning mix samples 3 and 4 compared favourably with the casein diet (control). Therefore, it would be an appropriate weaning food for infants or be used to complement the foods of infants.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML