-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2021; 11(1): 18-23
doi:10.5923/j.fph.20211101.02
Received: May 6, 2021; Accepted: May 17, 2021; Published: May 26, 2021

Nutritional Quality and Phytochemical Assessment of Some Under-Utilized Traditional Green Leafy Vegetables
Paulina O. Adeniji1, G. G. Daramola2, B. A. Salau2
1Department of Tourism Studies, Redeemer’s University, Ede, Osun State
2Department of Chemical Sciences, Redeemer’s University, Ede, Osun State
Correspondence to: Paulina O. Adeniji, Department of Tourism Studies, Redeemer’s University, Ede, Osun State.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The possible relevance of under-utilized vegetables for nutrition; has been reported in some studies. This study probed proximate, mineral and phytochemical properties three under-utilized traditional leafy vegetables; Solanum nigrum (SN), Cucurbita pepo (CP) and Crassocephalum crepidioides (CC). Standard methods were applied to determine proximate, macro, micro-minerals, phytate, tannin, saponin, alkaloids and glycosides. SN had the highest protein (17.50 ± 0.38%), Magnesium (65.04mg), CP had highest crude fiber (18.82 ± 0.26%), Calcium (133.54mg) and Iron (5.80mg) while CC had highest fat (11.65 ± 0.66%) and ash content (11.20 ± 0.17%) and Potassium (76.21mg). CC had a higher proportion of all phytochemicals compared to SN and CP. The vegetables were generally rich in minerals of nutritional relevance and show that if fully adopted into the diet could provide a boost to health. CC may need further food processing to denature its antinutrient factors before its nutritional content can be of benefit if consumed.
Keywords: Solanum nigrum, Cucurbita pepo, Crassocephalum crepidioides, Leaves, Nutrition
Cite this paper: Paulina O. Adeniji, G. G. Daramola, B. A. Salau, Nutritional Quality and Phytochemical Assessment of Some Under-Utilized Traditional Green Leafy Vegetables, Food and Public Health, Vol. 11 No. 1, 2021, pp. 18-23. doi: 10.5923/j.fph.20211101.02.
Article Outline
1. Introduction
- The use of traditional green leafy vegetables for food and therapeutic purposes is still popular in many African settings. The most common vegetables are the edible ones which are usually grown in- home/commercial gardens and are mainly prepared into soups and sauces to be eaten with common starchy staples. The lesser widespread ones have lesser contribution to the diet and are more utilized for therapeutic purposes over a wide range of illnesses. It is a well-known fact that vegetables are rich sources of nutrients and phytochemicals (Guerrero et al., 1998; Young, and Woodside 2001; Gupta et al., 2005) [1,2,3] and despite the vast diversity available within the African flora for utilization, several species can still be termed as under-utilized, which means they are not widely adapted as either food or source of therapy. Over the years, several vegetables which were popular vegetables in rural areas have also lost significance in the urban diets for reasons of urbanization and nutrition transition (Ejoh et al., 2019). [4] The possible relevance of these under-utilized vegetables has been reported in some studies, which found that some of them could provide better nutrition compared to their more commonly consumed counterparts (Gupta et al., 2005; Ebert, 2014). [3,5] Another possible advantage discovered with this under-utilized species are the wild nature of their cultivation which implies little or no effort in their production (Edmonds, and Chweya, 1997; Ayodele, 2004). [6,7] Considering the availability and affordability of leafy vegetables in most African food systems, and their potentials as rich sources of macro- and micronutrients, antioxidants and other beneficial phytochemicals, it is inevitable that they will still remain a sustainable solution to the persistent problem of micronutrient deficiencies also called hidden hunger in Sub-Saharan Africa. While the research interest in the food composition of under-utilized leafy vegetables has been reported extensively, further probes of under-utilized species have been recommended for better understanding of the existing rich diversity of leafy vegetables (Flyman and Afolayan, 2006) [8]. Based on this assertion, this study selected three species of under-utilized leafy vegetables (Solanum nigrum, Cucurbita pepo, and Crassocephalum crepidioides) for a probe of their nutritional, mineral and phytochemical properties so as to ascertain their sustained use beyond therapeutic purposes and their possible full-scale adaptation to the diet. The leaf of Solanum nigrum, also referred to as black nightshade is a common widespread vegetable that grows extensively in Africa and the Americas (Edmonds and Chweya, 1997) [6]. The leaves which are also referred to as ‘efo odu’ in the southwestern part of Nigeria have been reported to be a good source of plant protein and substantial anti-oxidant properties (Akubugwo et al., 2007). [9] Their mineral content has however not been fully explored. The leaves of Cucurbita pepo are the foliage parts of pumpkin, which are also useful for its seeds and fruits. In local parlance around southwestern Nigeria, its leaves are popularly called ‘efo elegede’. Its pulp and leaves have been identified to possess medicinal use (Ayodele, 2005) [7]. Reports on the nutrient content of the leaves have also been presented (Aruah et al., 2011; Oloyede, 2012; Fadupin et al., 2014). [10,11,12] Despite this information, the fuller understanding of its mineral and phytochemical components is still lacking. The flower Crassocephalum crepidioides also called Okinawa Spinach, Redflower Ragleaf, or Fireweed is locally referred to as ‘Ebolo’ in southwestern Nigeria. The leaves have found use in preparation of soups in Africa and beyond (Ajibesin, 2008). [13] It has also found extensive therapeutic use such as treatment for indigestion and headache in Nigeria, stomach upset in Congo and to treat sleeping sickness in Tanzania (Denton, 2004). [14] A report presented by and Adanlawo, 2007 [15] characterized its nutrient composition especially its amino acid profile and established its ability to supply needed nutrients. However, this report did not reveal its full mineral and phytochemical potential. The selected species have been earlier reported to be a part of diets even though in under-utilized manners (Osewa et al., 2013; Adepoju and Aka, 2019) [16,17] and also useful for therapeutic purposes (Adebooye and Opabode 2004; Ayodele, 2005) [18,7] but some gaps still exist in their mineral composition and phytochemical properties which could provide more specific information about the usefulness of the selected species.
2. Materials and Methods
- Two under-utilized indigenous vegetables Solanum nigrum (efo odu) and Cucurbita pepo (efo ebolo), were obtained in Gbogan and Ode Omu from hawkers while Crassocephalum crepidioides (efo elegede) was planted and harvested for the purpose of this study by the researcher in Ede, Osun State, Nigeria. The three vegetables were taken to the chemistry laboratory in Redeemer’s University, Ede, Osun state Nigeria for chemical analysis.Proximate analysisDetermination of moisture content:The weight of an empty aluminum dish can was determined before the sample was introduced into it. About 2g of the sample was weighed out and further weighed in the aluminum dish can. The aluminum dish can was then dried in a hot air oven at the temp of 105°C for 24 hours and cooled in a desiccator and weight measured. Determination of ash content:The method reported in literature AOAC, 2005 [19] was used. An empty clean crucible dish was weighed. The 2g of the sample was weighed in the empty clean crucible; the weight was noted. The crucible containing the sample was further placed in a muffle furnace and ashed at 500°C - 600°C for 3 hours. The crucible was removed from the muffle furnace and cooled in a desiccator, and weighed using a weighing balance. The ashed sample was preserved for minerals analysis.Determination of Mineral CompositionThe ashes obtained from determination of Ash Content were dissolved in HNO3, the solution was filtered and analyzed according to AOAC (2005) [19] using an Atomic Absorption spectrophotometer.Determination of crude fibre contentAs reported in literature AOAC, 2005 [19] was used. 2g sample were weighed in a 1L conical flask and the weight noted. 200mls of boiling 1.25% H2SO4 were added into the conical flask containing the sample and boiled gently for 50minutes. The content was filtered through a muslin cloth and the residue scraped into a clean conical flask using a spatula. 20mls of boiling 1.25% NaOH were added and allowed to boil gently for 30 minutes. Further filtration was done using a Buckner pressurized filter through a muslin cloth spread over its funnel. The resulting residue was thoroughly washed with distilled water and rinsed once with 10% HC1 and twice with ethanol. Final rinsing with petroleum ether was done thrice and allowed to drain. The residue was scraped into a flat silica dish and dried overnight in the oven at 105°C. Afterwards, it was cooled in the desiccators and the sample weighed. The sample was further ashed at 55°C for 90 minutes in a muffle furnace, cooled again in the desiccators and reweighed. Percentage fibre content was then determined as follows: Determination of Fat content The method reported by AOAC, 2005 [19] was used. A 250ml round bottom flask was dried in an oven, allowed to cool in the desiccators and the weight measured. 200ml of n-hexane were introduced into the dry 250ml round bottom flask. 75g of the sample was weighed and ramped with filter paper in a thimble. The thimble was placed into the soxhlet extractor, fitted into the round bottomed flask. The extraction apparatus was set up with the flask on the hot water bath of the soxhlet extracting unit. The content of the round bottom flask was then heated. As the methanol evaporated, it condensed dropped into the thimble where it extracted the methanol soluble constituent into the round-bottomed flask. The extraction lasted for 5-6 hours during which all the methanol moved up to the extractor, leaving behind the fats/oil in the round bottom flask. The porous thimble was removed with care and the methanol collected from the top for re-use. The round-bottomed flask containing the lipids was then removed and oven dried reconstitute through rotary evaporator for 1 hour at 105 - 110°C. After drying, it was further transferred into a desiccator (containing silica gel) where it was cooled and also weighed. Qualitative determination of phytochemicalsThe sample was analyzed for the presence of alkaloids, saponins, tannins, phenols, terpenes and cardiac - glycosides using standard protocols (Trease and Evans, 1989; Sofowora, 1993) [20,21] follows: 1ml of 1% HCl was added to 3ml of the prepared extract in a test tube. The mixture was heated for 20 minutes, cooled and filtered then the filtrate was used to test for the presence of the following chemical components:i. Alkaloids: 2 drops of Meyer’s reagent were added to 1ml of the filtrate. A reddish-brown precipitate indicates the presence of alkaloids.ii. Saponins: 5 drops of olive oil were added to 3ml of the filtrate in a test tube and shaken vigorously. The formation of a stable emulsion indicates the presence of saponins.iii. Tannin: 1ml of freshly prepared 10% potassium hydroxide (KOH) in a beaker, 0.5g of the extract was added and shaken to dissolve. A dirty precipitate observed indicates the presence of tannin.iv. Glycosides: The residue was re-dissolved in water in the water bath. Two milliliters of the solution in a test tube was added 10ml each of Fehling’s solution A and B. The mixture was shaken and heated in a water bath for 10 min. A brick-red precipitate indicates the presence of Glycosides.v. Determination of Total flavonoid content: 3ml of 1%Aluminium chloride solution were added to 5ml of each extract. A yellow coloration was observed indicating the presence of flavonoids.5ml of dilute ammonia solution were added to the above mixture followed by addition of concentrated H2SO4. A yellow coloration disappeared on standing. The yellow coloration which disappeared on standing indicates a positive test for flavonoids.Photochemical analysis of sampleAlkaloids:Materials and Reagent: Ethanol, H2S04, 0.5% Formaldehyde and Spectrophotometer, funnel and filter paper.Procedure: 0.5g of the sample was dissolved in 80% ethanol- 20% H2S04. One milliliter of the filtrate was added to 5ml of 60% H2S04 and allowed to stand for 5mins. Then, 5ml of 0.5% formaldehyde was added and allowed to stand for 3hrs.The reading was taken at absorbance of 565nm (Harborne, 1976). [22]Flavonoids:Materials and Reagent: HCl, Ethyl acetate, l% NH3, spectrophotometer, funnel and filter paper.Procedure: Flavonoids in the test sample was determined by the hydrolysis of spectrophotometric method. 0.5g of processed plant sample was mixed with 5ml of dilute HCl and boiled for 30mins. The boiled extract was allowed to cool and filtered. 1ml of the filtrate was added to 5ml of ethyl acetate and 5ml of 1% NHS. This was then scanned from 420nm - 520nm for the absorbance (Harborne, 1976). [22]Saponins:Materials and Reagent: NHCL, Petroleum ether, Acetone, Ethanol, Ferrous sulphate, H2SO4, spectrophotometer.Procedure: 0.5g of the sample was added to 20ml of IN HCI and was boiled for 4hours. After cooling it was filtered and 50ml of petroleum ether was added to the filtrate for ether layer and evaporated to dryness. 5ml of acetone ethanol was added to the residue. 0.4mls of each was taken into 3 different test tubes. 6ml of Ferrous sulphate reagent was added into them followed by 2ml of cone H2S04. It was thoroughly mixed after l0mins and the absorbance was taken at 490nm (Harborne, 1976). [22]Tannins:Materials and Reagent: Methanol, FeC13, NH4CI, K2Fe(CN)6, Spectrophotometer, funnel and filter paper.Procedure: 0.5g of the grounded sample was shaken constantly for 1min with 3ml of methanol in a test tube and then poured into a Buchner funnel with the suction already turned on. The tube was quickly rinsed with an additional 3ml of methanol and the content poured at once into the funnel. The filtrate was mixed with 50ml of water and analyzed within an hour for aqueous extraction, 5ml of water was used for the extraction and for the rinse and the filtrate was added to 50ml of water 3ml of 0.1M FeCl3 in 0. 1NH4CI was added to 5ml of the extract and followed immediately by timed addition of 3ml of 0.008M potassium ferrocyanide. Absorbance was then taken at 720nm spectrophotometrically (Onwuka, 2005). [23]
3. Results and Discussion
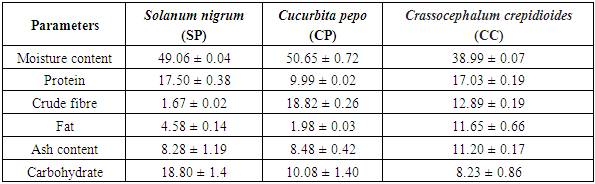
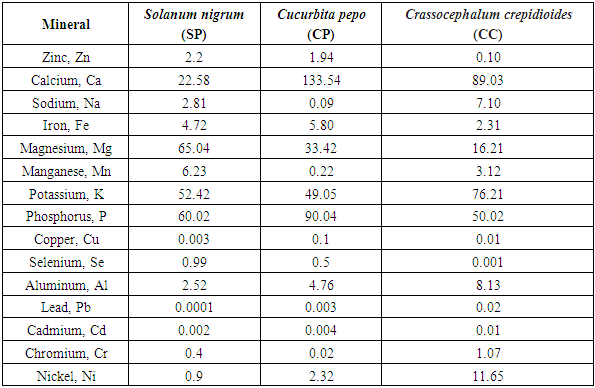
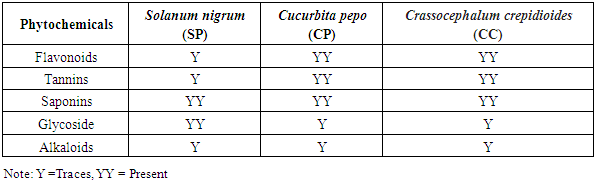
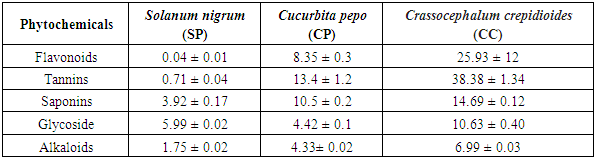
- The proximate composition of the analyzed samples is presented in Table 1. A similar range of proximate content has been reported by Akubugwo et al., 2007 and Aruah et al., 2011, [9,10] and for SN, and CP respectively. The highest value of moisture content was found in Curbita pepo (CP) which had 50.65 ± 0.72g followed by Solanum nigrum (SN) which had 49.06 ± 0.04g and the least moisture content value was found in Crassocephalum crepidioides (CC) with 38.99 ± 0.07g. The protein content found in SN (17.50 ± 0.38%) and CC (17.03 ± 0.19%) are relatively similar while that of CP (9.99 ± 0.02%) is lower. A urther probe of the amino acid composition of CC by Dairo and Adanlawo, 2007 [24] revealed that essential amino acid was insufficient which points to a need to further supplement CC with other protein sources despite the high protein content. Therefore, even though protein content seems to be high, caution may be needed in relying on these vegetables as sources of dietary protein. The crude fibre value was least in SN (1.67 ± 0.02%) and highest in CP (18.82 ± 0.26). The crude fibre content which suggests the ability of the vegetable to serve as roughage in food shows that SN may not be able to provide such benefits when compared with the CP and CC. CC had the highest fat content of 11.65 ± 0.66% while the highest carbohydrate content was found in SN (18.80 ± 1.4%) suggesting that SN may be fairly considered a relatively good source of digestible carbohydrate. The fiber, protein and carbohydrate values of SN presented in this study were found to be lower than those reported by Dhellot et al., 2006 [25] on SN within East Africa but were consistent with reports of Akubugwo et al., 2007 [9] on SN sampled in Nigeria. These differences may have resulted from varietal differences based on the geographical locations on which the two studies were carried out. The ash content values are relatively similar in SN (8.28 ± 1.19%) and CP (8.48 ± 0.4 2%) but higher in CC (11.20 ± 0.17%). The ash content is an estimate of the inorganic content of food samples. Since ash represents inorganic constituents mainly consisted of minerals, several factors may play out in this component of plant materials. These include the availability of such minerals in the soil on which the vegetables were grown, the translocative capacity of the vegetable as well as their bio-accumulative capacity. The results for minerals presented in Table 2 which can be grouped into essential macro minerals (Zinc, Iron, Calcium, Potassium Sodium), micro minerals (Magnesium, Manganese, Phosphorus, Aluminum, Copper, Selenium) and heavy metals (Lead, Cadmium, Chromium and Nickel) confirm this variance in inorganic constituents despite similar ash components. The results show that Calcium (22.58-1333.54mg/100g), Iron (2.31-5.80mg/100g), Magnessium (16.21-65.04mg/100g), Phosphorus (50.02-90.04mg/100g Th) and Potassium (95.42-76.21mg/100g) values for the vegetables were comparable and sometimes higher than values presented for other common traditional vegetables reported elsewhere (Gupta et al., 2005; Ebert, 2014; Ejoh et al., 2019). The significance of these minerals are numerous because they are required by many enzymes as co-factors and contribute to intracellular and extracellular functions in the body. The values of the heavy metals generally revealed a low concentration which indicates there is safety in consuming the vegetables. The phytochemical qualitative and quantitative analyses of the vegetables are presented in Tables 3 and 4 respectively. Alkaloids were found in trace amount in all the samples as shown in Table 3 while saponins were in appreciable amount. As presented in Table 4, the values obtained for CC is higher for all the phytochemicals compared to SN and CP. Phytochemicals may be considered as biologically active non-nutrient substances in the vegetables and may be responsible for antioxidant and medicinal activities exhibited by these vegetables. Conversely, these chemicals are also responsible for anti-nutrient factors in plant materials. Of all the phytochemicals considered in this study, flavonoids are beneficial. Flavonoids have been reported to have a high pharmacological and biochemical potential including radical scavenging property when contained in high proportions in a plant material (Bravo, 1998) [25]. However, saponin, tannin and alkaloids are anti-nutrient factors which interfere with metabolism of key nutrients and negatively influence their bioavailability. This attribute is however useful in their roles as medicinal and therapeutic uses against a large number of illnesses (Okwu and Emenike, 2006). [26] The flavonoid content presented in this study were consistent with results reported by Akubugwo et al., 2007 [9] on SN but lower in CP as reported by Oloyede, 2012. [11] The saponin, tannin and alkaloid content values were agreeable with results presented by Fadupin et al., 2014 for CP but higher than results by Aruah et al, 2011 [10]. The composition of glycosides in CC was highest when compared with SN and CP which indicates a high cyanogenic potential in its leaves.
|
|
|
|
4. Discussion
- When the results of this study were compared with literature, a similar range of proximate content had been reported for SN (Akubugwo et al., 2007), [9] and CP (Aruah et al., 2011) [10]. The values reported in this study were however lower than those reported elsewhere (Dairo, and Adanlawo, 2007). [24] A further probe of the amino acid composition of CC (Dairo, F.A.S. and Adanlawo, 2007) [24] revealed that essential amino acid was insufficient which points to a need to further supplement CC with other protein sources despite the high protein content. Therefore, even though protein content seems to be high, caution may be needed in relying on these vegetables as sources of dietary protein. The fiber, protein and carbohydrate values of SN presented in this study were found to be lower than those reported on SN within East Africa (Dhellot et al., 2006) [25] but were consistent with literature on SN sampled in Nigeria (Akubugwo et al., 2007) [9]. These differences may have resulted from varietal differences based on the geographical locations on which the two studies were carried out. The ash content is an estimate of the inorganic content of food samples. Since ash represents inorganic constituents mainly consisted of minerals, several factors may play out in this component of plant materials. These includes the availability of such minerals in the soil on which the vegetables were grown, the translocative capacity of the vegetable as well as their bio-accumulative capacity. The results show that Calcium (22.58-133.54mg/100g), Iron (2.31-5.80mg/100g), Magnesium (16.21-65.04mg/100g), Phosphorus (50.02- 90.04mg/100g) and Potassium (52.42-76.21mg/100g) values for the vegetables were comparable and sometimes higher than values presented for other common traditional vegetables reported elsewhere (Ebert, 2014; Gupta et al., 2015; Ejoh et al., 2019). [5,4] The significance of these minerals are numerous because they are required by many enzymes as co-factors and contribute to intracellular and extracellular functions in the body. The values of the heavy metals generally revealed a low concentration which indicate there is safety in consuming the vegetables. Phytochemicals may be considered as biologically active non-nutrient substances in the vegetables and may be responsible for anti-oxidant and medicinal activities exhibited by these vegetables. Conversely these chemicals are also responsible for anti-nutrient factors in plant material. Of all the phytochemicals considered in this study, flavonoids are beneficial. Flavonoids have been reported to be a have high pharmacological and biochemical potential including radical scavenging properties when contained in high proportions in a plant material (Bravo, 1998) [26]. However, saponin, tannin and alkaloids are anti-nutrient factors which interfere with metabolism of key nutrients and negatively influence their bioavailability. This attribute is however useful in their roles as medicinal and therapeutic uses against a large number of illnesses (Okwu and Emenike 2006). [27] The flavonoid content presented in this study were consistent with literature results on SN (Akubugwo et al., 2007) [9] but lower in CP (Oloyede 2012) [22]. The saponin, tannin and alkaloid content values were agreeable with results presented by a report on CP (Fadupin et al., 2014) [12] but higher than reported results (Aruah et al., 2011). [10] The composition of glycosides in CC was highest when compared with SN and CP which indicates a high cyanogenic potential in its leaves.
5. Conclusions
- This probe shows a wide range in the proximate, mineral and phytochemical content of the selected under-utilized vegetables. While one vegetable could not be singled out as having the most favorable composition, the vegetables were generally rich in minerals of nutritional relevance and show that if fully adopted into the diet could provide a boost to health. This trend was also reflected in the composition of heavy metals which showed low concentration across the selected vegetables. Overall, CC had a higher proportion of all phytochemicals analyzed in this study which implies that it may need further food processing to denature its anti-nutrient factors before its nutritional content can be of benefit if consumed.Suggestions and improvements for further studies:Nutritional trials on animals and humans could be carried out with clinical profile such as appearance, body weight including chemical analysis of urine, blood etc. These vegetables have been in existence but are under-utilized. People should be encouraged to plant these vegetables in their various home-gardens and introduce them to their neighbors. Declarations of interest: none
Funding Statement
- This research did not receive any specific rant from funding agencies the public, commercial, or not-for-profit sectors.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML