-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2020; 10(3): 72-80
doi:10.5923/j.fph.20201003.03
Received: Oct. 3, 2020; Accepted: Oct. 16, 2020; Published: Oct. 26, 2020

Enhancing the Stability of Polyunsaturated Sesame Oil Against Oxidation via Addition of Natural Antioxidant During Storage
Ulfat M. Omar1, Hadeil M. Alsufiani1, 2, Arwa A. Almalki1, Amjad S. Alsolami1, Lujain W. Kuddah1, Nourah M. Alghashmari1, Tahany Saleh Aldayel3, Rasha A. Mansouri1
1Department of Biochemistry, Faculty of Sciences, King Abdulaziz University, Jeddah, Saudi Arabia
2Experimental Biochemistry Unit, King Fahad Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
3Nutrition and Food Science, Department of Physical Sport Sciences, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia
Correspondence to: Rasha A. Mansouri, Department of Biochemistry, Faculty of Sciences, King Abdulaziz University, Jeddah, Saudi Arabia.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Punicalagin is a natural compound that extracted from pomegranate husk and can be used as valuable by product. Antioxidant effect of punicalagin on the oxidative stability of sesame oil upon storage was assessed and compared with synthetic antioxidant butylated hydroxy tolouene (BHT). Sesame oil sample was categorized into three groups, sesame oil alone, sesame oil with BHT and sesame oil with punicalagin. All samples monitored and tested throughout 60 days. Tested experiments involved peroxide value (PV) to identify the primary products of oil oxidation, Thiobarbituric Acid Reactive substances (TBARS) and P-Ansidine value (PAV) both to identify the secondary products of oxidation. Moreover, total oxidation index (TOTOX) was calculated to evaluate the overall oxidation status of sesame oil samples.The results at day 30 and 60 revealed that the presence of punicalagin with sesame oil significantly reduced PV, PAV and TOTOX while it significantly inhibited TBARS formation at day 60 only in comparison with sesame oil alone. Moreover, the results of sesame oil with BHT exhibited significant reduction in PAV and TOTOX when compared to oil with punicalagin. These findings indicated a substantial potential for using punicalagin as natural antioxidant to improve the quality of sesame oil during storage.
Keywords: Natural antioxidant, Oxidation, Punicalagin, Sesame, Oil
Cite this paper: Ulfat M. Omar, Hadeil M. Alsufiani, Arwa A. Almalki, Amjad S. Alsolami, Lujain W. Kuddah, Nourah M. Alghashmari, Tahany Saleh Aldayel, Rasha A. Mansouri, Enhancing the Stability of Polyunsaturated Sesame Oil Against Oxidation via Addition of Natural Antioxidant During Storage, Food and Public Health, Vol. 10 No. 3, 2020, pp. 72-80. doi: 10.5923/j.fph.20201003.03.
Article Outline
1. Introduction
- Sesame oil is an edible vegetable oil that extracted from raw sesame seed (Sesamum Lndicum Linn). Sesame oil is ranked as the second after olive oil due to its nutritional value. [1] Sesame oil is known for its usage as cooking oil due to its oxidative stability at storage [2,3] and during frying [4] compared to other vegetable oils. The remarkable stability of sesame oil is due to the presence of different antioxidant compounds such as tocopherols and lignans [5,6]. The common antioxidant activity of sesame oils’ tocopherol was showed by 𝛾-tocopherol [7] and three lignan compounds including sesamin, sesamolin and sesamol are responsible for the strong antioxidant property of sesame oil [8]. Moreover, sesame oil contains different fatty acids such as linoleic acid (35-50%), oleic acid (35-50%), palmitic acid (7-12%) and stearic acid (3.5-6%) [9]. The composition and ratio of fatty acids in sesame oil are affected by physiological and ecological factors [10]. Therefore, sesame oil was selected in this study.Oil is easily oxidized when exposed to different factors such as presence of light, trace metal and oxygen due to presence of unsaturated bond in its chemical structure. Oxidation of oil affect adversely on nutritional quality and shelf life of the oil [11]. Lipid peroxidation is a complex process which occurs in stages and rapidly produce numerous compounds. These compounds are responsible for the rancidity of lipids [12,13]. Hydroperoxides consider one of the main primary oxidation products which oxidized further during the process to produce secondary oxidation products. Different organic compounds as alkanes, alkenes, aldehydes and ketones are examples for the second oxidation products. In order to minimize the occurring of lipid oxidation and prolong the shelf-life, antioxidants were used. Antioxidants prevent the autoxidation of oils and fats by transferring their hydrogens to free radicals [14] and hence reduce the possibility of oil oxidation and rancidity. Antioxidants can be either presence in nature specifically in plant kingdom (e.g. Gallic acid, α-tocopherol) or manufactured (e.g. butylated hydroxy anisole and BHT) [15]. Although synthetic antioxidants succeeded to highly minimize lipid oxidation but there is a safety concern on health for using them. Thus, attention is now increasingly paid to the utilization of more effective and non-toxic antioxidants of natural origin. Researchers have been found natural antioxidant can represent similar action to the synthetic one without adverse effect on consumer health [16]. One of the highly natural antioxidant sources is pomegranate, due to the presence of hydrolysable tannin compounds [17]. The waste products that result after peeling pomegranate fruit are potentially valuable by-products as they contain a substantial amount of punicalagin [18]. Punicalagin (Figure 1) is the main component of pomegranate husk and one of the hydrolysable tannins in Pomegranate fruit in addition to gallic and ellagic acid. It has been reported to have a range of health benefits in vitro and in animal studies, including anticancer, antiinflammation, antibacterial, hepatoprotective activity and antioxidant [44-46]. Furthermore, punicalagin having the capacity of free radical scavenging that could stop lipid oxidation chain reactions [47]. The potential understanding of antioxidant effect of punicalagin may well lead to the utilization of this waste by product compound as a source of natural antioxidant. Therefore, the current study will evaluate the effect of punicalagin on the oxidation stability of sesame oil during storage in order to improve the oil quality and comparing the results with synthetic antioxidant BHT.
2. Materials and Methods
2.1. Preparation of Oil Samples
- Fresh Sesame seeds (Sesamum Indicum Linn) were obtained from Jizan city, Saudi Arabia (SA). The seeds were then given to Albarkah factory in Jeddah city, SA to process and to extract the oil. Extracted oil was categorized into 3 different samples: sesame oil alone (control), sesame oil treated with synthetic antioxidant BHT and sesame oil treated with natural antioxidant Punicalagin. The concentration of punicalagin and BHT based on (Shah et al, 2016). Oil samples were prepared according to Rashid et al. [19] with slight modifications. Briefly, stock solutions of punicalagin and BHT was firstly prepared with concentration of 600 ppm by dissolving 10 mg of each in 16.6 ml pyrogallol. Punicalagin and BHT were obtained from Sigma Aldrich (St. Louis, MO). Each antioxidant was then added to 1 L sesame oil. All 3 samples were stored for 60 days at laboratory temperature (25C) and exposed to atmospheric oxygen and light. To investigate the progression of oil oxidation and the effect of both antioxidants, all experiments were conducted at 0, 30 and 60 days. All analytical determinations were performed in triplicate manner to obtain independent measurements for every time.
2.2. Peroxide Value (PV)
- Peroxide value is used to indicate the concentration of peroxides and hydroperoxides that are formed in the initial stages of lipid oxidation process [20]. When the oil start to oxidize, peroxides start to form leading to iodine liberation from potassium iodide. Iodine formed can be then estimated by titration with standard sodium thiosulfate using the method of Horwitz et al. [21]. Briefly, 5 g of oil sample was placed into 250 ml flask, followed by adding 30 ml acetic acid-chloroform solution and shaking until the oil completely dissolved. After dissolving, 0.5 ml saturated potassium iodide solution was added and swirled for exactly 1 min followed by an immediate addition of 30 ml Deionized water. One ml of starch was added as an indicator. Sample was then titrated against 0.1 N sodium thiosulfate until the blue-gray color disappeared. The volume of titrant was recorded and used to calculate the peroxide value according to the following equation and results were expressed as milliequivalents of peroxide/ Kg oil:
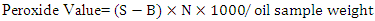 Where, S = Volume consumed during titration of the sample (ml)B = Volume consumed during titration of the blank (ml) N = Normality of sodium thiosulfate solution
Where, S = Volume consumed during titration of the sample (ml)B = Volume consumed during titration of the blank (ml) N = Normality of sodium thiosulfate solution 2.3. Acid Value (AV)
- Acid value experiment was used in this study to calculate the percentage of free fatty acid liberated due to the hydrolysis of fat (triglycerides) using the method of AOCS [22]. Briefly, in 250 ml flask, 1 g oil was added to 10 ml ethanol for dissolving. Following by addition of 2 drops of phenolphthalein as indicator. Oil samples were then titrated against 0.1 N potassium hydroxide (KOH) until the faint pink color appeared. The volume of titrant was recorded and used to calculate the free fatty acid value according to the following equation:
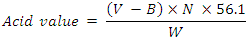 Where,V = titrant volume consumed during titration of the sampleB = titrant volume consumed during the titration of the blankN = normality of KOHW = weight of oil (g)
Where,V = titrant volume consumed during titration of the sampleB = titrant volume consumed during the titration of the blankN = normality of KOHW = weight of oil (g)2.4. Thiobarbituric Acid Reactive Substances (TBARS Value)
- In this method Malondialdehyde (MDA) that produced from lipid hydroperoxide decomposition will form red complex. The absorbance of this red chromophore can be measured at 532 nm using a spectrophotometer [23]. Firstly, a 50 microliter of oil samples were drawn with a microsyringe then added to test tubes containing a mixture of 0.8 ml of distilled water, 0.2 ml of 8.1% sodium dodecyl sulphate (w/v), 1.5 ml of 20% acetic acid (w/v) with pH 3.5 and 1.5 ml of 0.8% 2-thiobarbituric acid solution in water (w/v). Samples were then heated at 100C for 1 hr. After cooling, all samples were centrifuged at 4300 x g for 10 min. Finally, the absorbance of the upper layer was measured at 532 nm using a spectrophotometer. The concentration of MDA in each sample was then determined by comparing the average optical density of samples with a standard curve of Tetraethoxypropane (TEP). Stock solution of 1 μg/ml TEP was prepared by adding 0.1 μg TEP to 9.999 ml distilled water. A standard curve was prepared by using serial dilution of TEP with concentration range: 0.2, 0.4, 0.6, 0.8 and 1 μg/ml. The absorbance of each concentration was measured and used to calculate the amount of MDA in oil samples, reported as μg MDA/ g oil.
2.5. P-Anisidine Value (PAV)
- PAV is used in this study to evaluate the secondary products of oil peroxidation. The principle of the method is built on the reaction between P-methoxy aniline (Ansidine) and aldehydic compounds that produced from the decomposition of hydroperoxide compounds. This method was performed according to Schwieter [24] with some modification. In brief, two solutions were prepared, solution A and B. Solution A contains 0.5 g oil sample that dissolved in 25 ml of 2, 2, 4-Trimethylpentane (iso-octane). Absorbance of solution A was then measured at 350nm using iso-octane as a blank. Solution B was prepared by adding 1 ml P-anisidine dissolved in glacial acetic acid (2.5 g/ L) to 5 ml of solution A. Solution B was mixed and stored against light. After 10 min exactly, the absorbance was measured at 350 nm using ratio of 1 ml: 5 ml P-anisidine and iso-octane respectively as a blank. The p- anisidine value calculated according to the following equation:
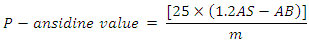 Where,
Where,

2.6. Total Oxidation Value (TOTOX)
- The overall oxidation index of all sesame oil samples was calculated using method of Pereira et al. [25] according to following equation:
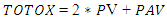 PV = peroxide Value PAV = P-anisidine Value
PV = peroxide Value PAV = P-anisidine Value2.7. Statistical Analysis
- Results were statistically analyzed using GraphPad Prism software version (8.1.0). Statistical analysis comparisons were made by two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Data were presented as means ± SEM and differences at p < 0.05 were considered to be significant.
3. Results
3.1. Peroxide Value (PV)
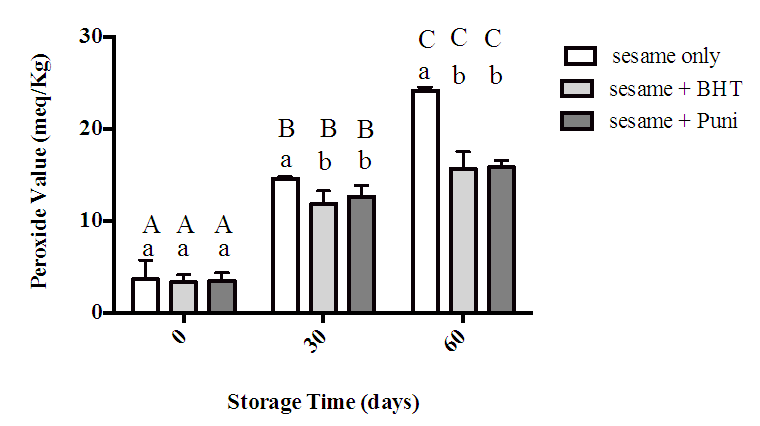
- Figure 2 demonstrates the PV for sesame oil alone (control), sesame oil with BHT and sesame oil with punicalagin under storage condition for 60 days. The results showed a significant increase in PVs for all samples as the storage time extended. At zero time, the result exhibited no significant difference between the control and sesame oil samples with BHT and Punicalagin individually. However, as storage period continued to 30 and 60 days, presence of BHT and Punicalagin was significant in reducing peroxide values when compared to the control. The results showed no significant difference in PVs values between treated samples; oil with BHT and sesame oil with punicalagin.
3.2. Acid Value (AV)
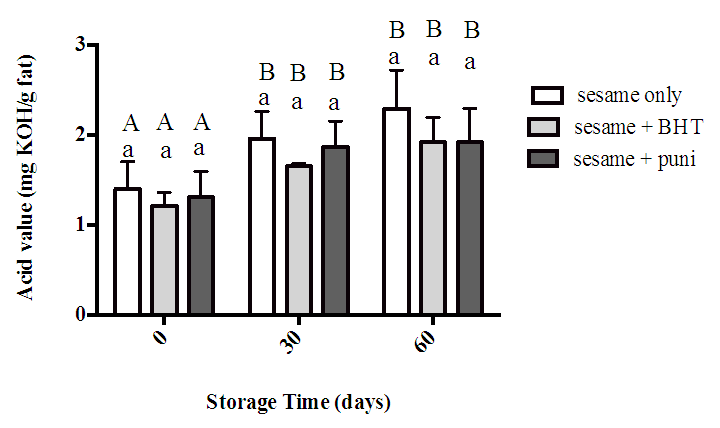
- The acid values for sesame oil alone (control), sesame oil with BHT and sesame oil with punicalagin under storage condition for 60 days are shown in Figure 3. At zero time, the result revealed that there was no significant difference between all samples. After 30 days the acid value of all samples increased in comparison with zero time but still no significant difference between all samples. As storage time continued to 30 then 60 days, the acid value of all samples increased in comparison with zero time but still no significant difference between all samples.
3.3. Thiobarbituric Acid Reactive Substances (TBARS Value)
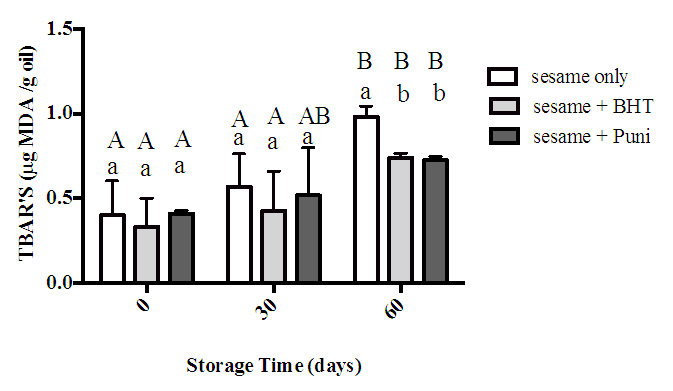
- Figure 4 illustrates TBARS value for control, sesame oil with BHT and sesame oil with punicalagin during storage for 60 days. Value of TBARS increased gradually in all samples with significant increase at day 60 only. In addition, the results at this time revealed that BHT and punicalagin treatments exhibited a significant reduction in TBARS values (0.75 μg MDA/g oil and 0.73 μg MDA/g oil; respectively) when compared to the control. However, the effect of punicalagin to reduce TBARS formation in sesame oil was not significantly different from the effect of BHT.
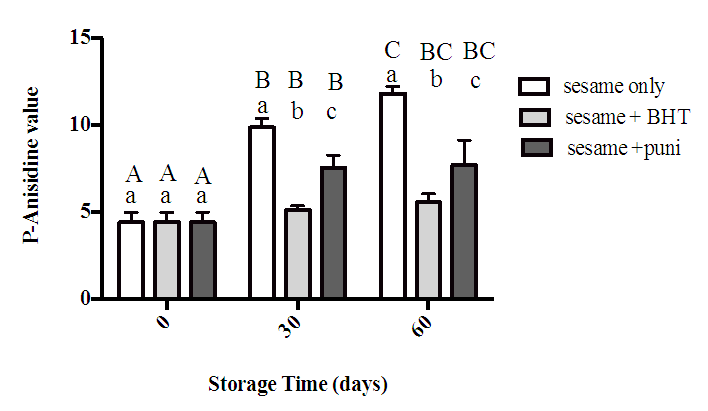
3.4. P-Anisidine Value (PAV)
- Data in Figure 5 represent the amount of p- anisidine that measured throughout 60 days. Over the storage time, the results showed that control sample exhibited the highest content of ρ‐anisidine followed by sesame oil with punicalagin then sesame oil with BHT. At zero time, the result exhibited no significant difference between all samples. At day 30 and day 60, although sesame oil with punicalagin showed significant reduction in PAV compared to the control, sesame oil with BHT exhibited the lowest reduction in PAV in comparing with other samples.
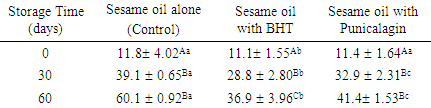
3.5. Total Oxidation Value (TOTOX)
- Table 1 shows the total oxidation changes over time for sesame oil alone (control), Sesame oil with BHT and Sesame oil with Punicalagin. The highest oxidation value was at day 60 of storage for all oil samples. At day 30 and 60, sample with BHT and sample with punicalagin exhibited significantly lower oxidation index when compared to the control. However, BHT revealed significant reduction in total oxidation of sesame oil when compared to oil with punicalagin.
4. Discussion
- The current study evaluated the effect of Punicalagin as a natural antioxidant extracted from waste material (pomegranate husk) on the oxidative stability of sesame oil during storage. The results obtained were compared with pure sesame oil and sesame oil treated with BHT. Different assays were applied as PV, FAA, TBARS, PAV and TOTOX to determine the activity of antioxidants used on primary and secondary oxidative compounds that produced during storage.
4.1. Peroxide Value
- Peroxide value was used to measure the primary oxidation product that are produced in fats upon oxidation [24] According to CODEX standard for oils, PV should be lower than (15 Meq/kg oil) [26]. The results obtained from this study indicated that PV of sesame oil alone (control) was within reference range at the start of the study (3.7 Meq/kg oil) and then increased gradually until it reached (24 Meq/kg oil) at the end of the study indicating rancidity of the oil. These results are in line with data reported recently by Pereira et al. [27] who found that PV of sesame oil exposed to oxygen and light for sixty days was 20 Meq/kg oil. Although sesame oil is considered a stable oil (due to the presence of tocopherols and lignans), the efficacy of these antioxidants was attenuated by the storage conditions (atmospheric oxygen and light). At zero time there were no significant difference between Sesame oil alone and Sesame oil treated either with BHT or Punicalagin. These result in concur with study done by FAO [28] which stated that there was no significant difference on oxidative stability of walnut oil treated with natural and synthetic antioxidants at zero time of storage. As storage time continued to 60 days, the control sample showed a significant difference compared to samples treated with BHT and Punicalagin. These results might be related to the addition of antioxidants which enhance the polyunsaturated structure of sesame oil [29] and provides protection against oxidation process [30,31]. Similar results were reported by Alsufiani et al. [32] who found that the addition of either punicalagin or BHT to canola oil decreased the peroxide values of the samples compared to control oil [32]. Also, found similar results when they add natural antioxidants to sunflower oil [33].
4.2. Acid Value
- The acid value experiment indicates the amount of free fatty acids in oil sample that liberated upon fat hydrolysis. It also determines the quality of sesame oil as the more acid value obtained, the more oil oxidized and hence had low quality [34]. In the present study, AV of all sesame oil samples were increased gradually with storage time but they were within reference range (4 mg KOH/ g oil) [26]. This increase in AV with time was previously reported by Salaheldeen et al. [27] in sesame oil as well as in other oils such as palm, peanut and sunflower.In this study sesame oil alone had no significant difference compared to sesame oil with BHT or punicalagin at all storage time points. This result was similar to the finding of another research when sunflower oil was treated with either natural or synthetic antioxidant [35].
4.3. TBARS Value
- In this study, TBARS method was used to evaluate the effectivness of punicalagin to inhibit the formation of secondary oxidation products and comparing the result with absence of antioxidant and presense of BHT as synthetic antioxidant. Continues accumilation of MDA was observed throughout the storage time among all samples in this study. This observation was noticed previously in sereval studies done by Alsufiani et al. [32], Iqbal et al. [36], Bardhan et al. [37]. Interestingly, presence of punicalagin in sesame oil sample successfully reduced MDA formation to 0.73 μg MDA/g oil when compared to sesame oil alone 0.98 μg MDA/g oil at day 60. Moreover, the reduction effect expressed by punicalagin was not significantly different in comparison with BHT. Similar results were stated by Mansouri [38] who studied the effect of punicalagin addition on olive oil stability through 60 days storage period. The study reported a significant reduction in MDA formation in the olive oil treated with punicalagin when compared with olive oil alone. It also showed that there was no significant difference between the BHT and punicalagin samples.
4.4. P-Anisidine Value (PAV)
- Researchers using P-Anisidine value to estimate the accurate oxidative rancidity of fats and oils with analyzing the secondary oxidation products of unsaturated fatty acids, mostly conjugated dienals and 2-alkenals [39]. The PAV of all sesame oil samples showed general increasing during the storage time. Similar results were obtained by other researchers when they estimated the oxidative process during storage of different oils such as canola, peanut, pecan and rapseed oils [32,40,41]. Adding punicalagin to sesame oil was significantly delayed the formation of secondary oxidation products to score 7.70 in comparison with 11.80 scored by sesame oil alone. The result obtained in this study is in concur with previous studies on canola oil conducted by Agregán et al. [20] , Shahbazi, and Shavisi [42]. Nevertheless, punicalagin’s effect that observed in this experiment was not as powerful as BHT in inhibiting alkanal formation. This agrees with the results of Mansouri [38], who reported that the addition of punicalagin on olive oil for 60 days did not show better results when compared to the oil sample with BHT. This observation could be explained by the chemical structure difference between BHT and punicalagin. The synthetic antioxidant (BHT) is known to have only one OH group attached to the aromatic ring. On the other hand, punicalagin possesses around 15 OH groups attached to several aromatic rings. Therefore, BHT showed better oil oxidation protective effect than punicalagin by providing more sites to bind free radicals [40]. Another possible explanation is the low concentration of punicalagin used in our study.
4.5. TOTOX
- To obtain an overall information of sesame oil oxidation stability, TOTOX was used as it includes both PV as primary oxidation and PAV as secondary oxidation [20]. At the end of study storage time, all samples exhibited an elevation in TOTOX index as expecting from the continuing in oxidation process. These findings are in concur with study done by Maszewska et al. [43] as they noticed significant elevation in TOTOX value of peanut, corn and rapeseed oils upon storage. In the current study, oil treated with Punicalagin and BHT significantly reduce the overall oxidation value to (41.41) and (36.89) respectively in comparison with sesame oil alone (60.14). A previous study conducted by Aloqbi et al. [18] to test the antioxidant activity of punicalagin in vitro showed that this compound has a significant radical scavenging activity and reducing power ability. Accordingly, we hypothesized that the effectiveness of punicalagin on overall oxidation of oil could be through these two mechanisms. To support this hypothesis, future studies should examine these mechanisms in oil samples.
5. Conclusions
- Different quantitative parameters such as PV, AV, TBARS, PAV and TOTOX were used in this study in order to examine the antioxidative effect of punicalagin as natural antioxidant on sesame oil during storage. Punicalagin significantly reduced the formation of primary and secondary oxidation products throughout storage period as indicated by reducing PV, TBARS, PAV and TOTOX when compared to sesame oil alone. Although BHT treatment exhibited significant better results, punicalagin showed promising results in protecting sesame oil from oxidation. According to these results, punicalagin can be a good candidate as natural antioxidant. However, further studies might be required to investigate the effect of punicalagin for longer storage time. In addition, examining the effect of higher concentration of punicalagin is also suggested.
ACKNOWLEDGEMENTS
- The research team would like also to thank Science Research and Innovation Unit in Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabi and mawakeb alajr for supporting this work.
Conflict of Interest
- The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
| [1] | Alpaslan M, Boydak E, Demirci M. Protein Content and Oil Composition of Soybean and Sesame Seeds Grown in the Harran (GAP) Area of Turkey. Research gate 2017. https://www.researchgate.net/publication/320805632. |
| [2] | Beroza M, Kinman ML. Sesamin, sesamolin and sesamol content of the oil of sesame seed as affected by strain, location grown, ageing and frost damage. J Am Oil Chem Soc. 1955; 32 (6): 348-50. |
| [3] | Namiki M. The chemistry and physiological functions of sesame. Food Rev Int. 1995; 11: (2), 281-329. |
| [4] | Downey RK, Robbelen G, Ashri A. Oil Crops of the World: Their Breeding and Utilization. Published by McGraw-Hill. Food and Agriculture Organisation of the United Nations. 1989. |
| [5] | Fukuda Y, Nagata M, Osawa T, Namiki M. Contribution of lignan analogues to antioxidative activity of refined unroasted sesame seed oil. J Am Oil Chem Soc. 1986; 63: (8): 1027-1031. |
| [6] | Moazzami AA, Kamal-Eldin A. Sesame seed is a rich source of dietary lignans. J Am Oil Chem Soc. 2006; 83 (8): 719-723. |
| [7] | Saldeen T, Li D, Mehta JL. Differential effects of alpha- and gamma-tocopherol on low-density lipoprotein oxidation, superoxide activity, platelet aggregation and arterial thrombogenesis. J Am Coll Cardiol. 1999; 34 (4): 1208‐1215. |
| [8] | Wan Y, Li H, Fu G, Chen X, Chen F, Xie M. The relationship of antioxidant components and antioxidant activity of sesame seed oil. J Sci Food Agric. 2015; 95 (13): 2571‐2578. |
| [9] | O’Connor RT, Herb SF. Specification of fatty acid composition for Identification of fats and oils by gas liquid chromatography. J Am Oil Chem Soc. 1970; 47: 186A-197A. |
| [10] | Uzun B, Ulger S, Cagirgan M. Comparison of determinate and Indeterminate types of sesame for oil content and fatty acid composition. Turk J Agric Forest. 2002; 26 (5): 269-74. |
| [11] | Gordon MH, Kourkimskå L. The effects of antioxidants on changes in oils during heating and deep frying. J sci food. Agric. 1995; 68. |
| [12] | Addis PB. Occurrence of Lipid Oxidation Products in Foods. Food Chem Toxicol. 1986; 24: 1021–30. |
| [13] | Gerrard JA. Protein cross-linking in food. Food Biochem Food Proc. 2006; 223-240. |
| [14] | Aguilar-Hernández, I, Iris NK, López-Luke T, Contreras-Torres, FF, Wold JP, Ornelas-Soto N. Surface enhanced raman spectroscopy of phenolic antioxidants: a systematic evaluation of ferulic acid, p-coumaric acid, caffeic acid and sinapic acid. Vibrat Spect, 2017; 89: 113–22. |
| [15] | Ali HM, Abo-Shady A, Sharaf Eldeen HA, Soror HA, Shousha WG, Abdel-Barry O. A, Saleh AM. Structural Features, Kinetics and SAR Study of Radical Scavenging and Antioxidant Activities of Phenolic and Anilinic Compounds. Chemy Cent J. 2013; 7 (1): 53. |
| [16] | Whysner J, Wang CX, Zang E, Iatropoulos MJ, Williams GM. Dose response of promotion by butylated hydroxyanisole in chemically initiated tumours of the rat forestomach. Food Chem Toxicol. 1994; 32 (30): 215-222. |
| [17] | Omar U, Aloqbi A, Yousr M, Howell N. Protective effects of punicalagin on caco-2 intestine cell line under oxidative stress caused by tert-butyl hydroperoxide. J Pharm Nut Sci 2015; 5 (4): 249–56. |
| [18] | Aloqbi A, Omar U, Yousr M, Grace M, Lila MA, Howell N. Antioxidant Activity of Pomegranate Juice and Punicalagin. Nat Sci. 2016; 8: 235-46. |
| [19] | Rashid A, Qureshi MZ, Raza SA, William J, Arshad M. Quantitative determination of antioxidant potential of Artemisia Persica. Analele UniversităŃii din Bucureşti. 2010; 19: 23-30. |
| [20] | Agregán R, Munekata PE, Domínguez R, Carballo J, Franco D, Lorenzoa JM. Proximate composition, phenolic content and in vitro antioxidant activity of aqueous extracts of the seaweeds Ascophyllum nodosum, Bifurcaria bifurcata and Fucus vesiculosus. Effect of addition of the extracts on the oxidative stability of canola oil under accelerated storage conditions. Food Res Int. 2017; 99 (3): 986-994. |
| [21] | Horwitz W, Chichilo P, Reynolds H. Official Methods of Analysis of (AOAC) International, 20th ed.; Edited by George W. Latimer, Jr. Published by AOAC International, Rockville, Maryland, USA. 2012. |
| [22] | AOCS Press. Fats and Oils Handbook. Edited by Bockisch, M; Published by AOCS Press. 2015. |
| [23] | Dasgupta A, Klein K. Antioxidants in Food, Vitamins and Supplements. Elsevier Inc.: San Diego, CA, USA. 2014. |
| [24] | Schwieter U. Á 401 Ñ Fats and Fixed Oils. 2009; 1-14. |
| [25] | De Abreu PDA, Losada, PP, Maroto J, Cruz JM. Natural antioxidant active packaging film and Its effect on lipid damage in frozen blue shark (Prionace Glauca). Innovat Food Sci Emerg Technol. 2011; 12 (1): 50–55. |
| [26] | FAO. Codex Standards for Fats and Oils from Vegetable Sources. 1999. [http://www.fao.org/3/y2774e/y2774e04.htm accesed 12-5-2020]. |
| [27] | Salaheldeen M, Satti A, Awadallah B. Storage and thermal behavior of some cooking oils consumed from the local market of Sudan. Int J Cheml Stud. 2019; 7 (5): 919–924. |
| [28] | Martínez ML, Penci MC, Ixtaina V, Ribotta PD, Maestri D. Effect of natural and synthetic antioxidants on the oxidative stability of walnut oil under different storage conditions. LWT - Food Science and Technology, 2013, 51 (1): 44-50. |
| [29] | Borchani C, Besbes S, Blecker Ch, Attia H. Chemical Characteristics and Oxidative Stability of Sesame Seed, Sesame Paste and Olive Oils. J Agr Sci Tech. 2010; 12: 585-96. |
| [30] | Coates PM, Betz JM, Blackman MR, Cragg GM, Levine M, Moss J, White JD. Encyclopedia of Dietary Supplements. Second Edition.; published by Informa Healthcare, Paul Street, london, Uk. 2010. |
| [31] | Orsavova J. Misurcova L, Ambrozova JV, VichaR, Mlcek J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int J Mol Sci. 2015; 16 (6): 12871-12890. |
| [32] | Alsufiani H, Mansouri R, Almalki A, Alsolami A, Kuddah L, Yamani R, Alghashmari N, Omar U. Effect of Punicalagin as natural antioxidant on the oxidative stability of Canola oil during storage. Egypt J Chem 2020; 63 (5). |
| [33] | Carelli AA, Franco IC, Crapiste GH. Effectiveness of added natural antioxidants in sunflower oil. Int J Fats Oil, 2005; 56 (4): 303–10. |
| [34] | Cornelius J A. Some technical aspects influencing the quality of palm kernels. J Sci of Food Agric. 1966; 17(2): https://doi.org/10.1002/jsfa.2740170201. |
| [35] | Iqbal S, Bhanger MI. Stabilization of sunflower oil by garlic extract during accelerated storage. Food Chem. 2007; 100 (1): 246–54. |
| [36] | Iqbal S, Haleem S, Akhta, M, Zia-ul-haq M, Akbar J. Efficiency of pomegranate peel extracts in stabilization of sunflower oil under accelerated conditions. Food Res Int. 2008; 41 (2): 194-200. |
| [37] | Bardhan J, Sahoo B, Chakraborty R, Raychaudhuri U. Effect of addition of rice bran oil extract on the stability of sunflower oil , sesame oil and their blends. Int Food Res J. 2014; 21: (6), 2293–98. |
| [38] | Mansouri RA. Oxidative Stabilization Of Olive Oil By Punicalagin During Storage. J Biochem Technol. 2020; 11(2): 10-6. |
| [39] | Wang H, Liu F, Yang L, Zu Y, Wang H, Qu S, Zhang Y. Oxidative stability of fish oil supplemented with carnosic acid compared with synthetic antioxidants during long-term storage. Food Chem, 2011, 128 (1): 93-99. |
| [40] | Zhang YY, Zhang F, Thakur K, Ci AT. Wang, H, Zhang JG, Wei ZJ. Effect of natural polyphenol on the oxidative stability of pecan oil. Food Chem Toxicol. 119: 489-495. https://doi.org/10.1016/j.fct.2017.10.001. |
| [41] | Li C, Tang Z, Huang M, Tao N, Feng B, Huang S. Antioxidant efficacy of extracts produced from pickled and dried mustard in rapeseed and peanut oils. J Food sci. 2012; 77: (4), C394-C400. https://doi.org/10.1111/j.1750-3841.2011.02606.x. |
| [42] | Shahbazi Y, Shavisi N. Effect of methanolic prosopis farcta extract on storage stabilization of canola oil. J food sci Technol. 2019; 56 (1) 420-427. |
| [43] | Maszewska M, Florowska A, Dłużewska E, Wroniak M, Marciniak-Lukasiak K, Żbikowska A. Oxidative stability of selected edible oils. Molecules, 2018; 23: (7), 1746. https://doi.org/10.3390/molecules23071746. |
| [44] | Voravuthikunchai, S., A. Lortheeranuwat, W. Jeeju, T. Sririrak, S. Phongpaichit and T. Supawita. 2004. Effective medicinal plants against enterohaemorrhagic Escherichia coli O157: H7. J. Ethnopharmacol. 94: 49-54. |
| [45] | Xu, X., Yin, P., Wan, C., Chong, X., Liu, M., Cheng, P., ... & Xu, J. (2014). Punicalagin inhibits inflammation in LPS-induced RAW264. 7 macrophages via the suppression of TLR4-mediated MAPKs and NF-κB activation. Inflammation, 37(3), 956-965. |
| [46] | SUN, Y. Q., Xin, T. A. O., MEN, X. M., XU, Z. W., & Tian, W. A. N. G. (2017). In vitro and in vivo antioxidant activities of three major polyphenolic compounds in pomegranate peel: Ellagic acid, punicalin, and punicalagin. Journal of integrative agriculture, 16(8), 1808-1818. |
| [47] | Gil, M. I., A. Tomas-Berberan, B. Hess-Pierce, D. M. Holcroft and A. A. Kader. 2000. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agri. Food Chem. 48: 4581-4589. |
| [48] | Shah, H., Khan, A. A., & Ullah, S. (2016). Utilization of pomegranate peel extracts to enhance the stability of sunflower oil. Pakistan Journal of Food Sciences, 26(4), 218-225. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML