-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2020; 0(1): 26-34
doi:10.5923/j.fph.20201001.04

Application of Supercritical Fluids in the Conservation of Bioactive Compounds: A Review
Adela C. Martinez, M. Angela A. Meireles
LASEFI/DEA/FEA (School of Food Engineering)/UNICAMP (University of Campinas), Cidade Universitária "Zeferino Vaz", R. Monteiro Lobato, Campinas, SP, Brazil
Correspondence to: M. Angela A. Meireles, LASEFI/DEA/FEA (School of Food Engineering)/UNICAMP (University of Campinas), Cidade Universitária "Zeferino Vaz", R. Monteiro Lobato, Campinas, SP, Brazil.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The precipitation from supercritical antisolvents (SASs) of natural compounds solutions has become an excellent alternative to improve the solubility and availability of poorly water-soluble compounds. In this technique, CO2 is used as a supercritical fluid that marks the processing as a green technology and is ideal for use in the food industry due to its nontoxicity, economy and easy separation of solutes. This article reviews the application of the SAS technique in the food industry and highlights the influential factors in the generation of micro- and nanoparticles.
Keywords: Micronization, Natural Compounds, SAS Technique, Coprecipitation
Cite this paper: Adela C. Martinez, M. Angela A. Meireles, Application of Supercritical Fluids in the Conservation of Bioactive Compounds: A Review, Food and Public Health, Vol. 0 No. 1, 2020, pp. 26-34. doi: 10.5923/j.fph.20201001.04.
Article Outline
1. Introduction
- Fruits, vegetables, and plants produce many secondary metabolites responsible for flavor, aroma, and color, which have shown beneficial effects for human health against cancer and other chronic diseases such as cardiovascular disease, diabetes, and aging [1]. Currently, some plants or extracts from them are commercially available as infusions, tablets or extracts [2], in this way the essential oils or secondary metabolites present, they could be degraded easily (by oxidation, volatilization, heating, light) if they are not protected of external factors because they are unstable and fragile volatile compounds [3]. To improve the availability and quality of natural compounds, powder formation has been currently used as a protective mechanism for these substances, for example, micronization or encapsulation techniques using supercritical fluids [4].The application of supercritical antisolvent techniques or the use of supercritical carbon dioxide (CO2) as an antisolvent can yield highly concentrated plant extracts. This approach is an alternative for the food industry because it is considered an environmentally friendly process and allows good control over product properties, especially particle size, particle size distribution, crystal habit and polymorphic form [5]. Moreover, avoiding the degradation and decomposition of active compounds by operating at reduced temperatures in light and in the absence of oxygen as well as the subsequent processing of extracts is not necessary to separate the organic solvents [6].The aim of this review is to summarize topics that are involved in the application of the Supercritical Antisolvent (SAS) process.
2. Natural Compounds
- Plants have a secondary metabolism that allows them to produce and accumulate compounds of diverse chemical nature, called natural compounds, which are grouped into four main classes: terpenes, phenolic compounds, glycosides and alkaloids [7]. Notably, not all secondary metabolites are found in all plants. Furthermore, they are found in small quantities and vary according to the genus, family or plant species; most of these natural compounds are found in a mixture of alcohols, ketones, aldehydes and one or more secondary metabolites called essential oils.According to Martelli and Giacomini [8], essential oils are complex volatile mixtures that composed of between 20 to 60 compounds at different some concentrations where two or three major components are present in relatively high concentrations (20 to 70%) compared to others. On the other hand, Xiao et al. [9] considered essential oils as containing two types of major components such as terpenes and aromatics, which are known for their high volatility and low residual generation. Examples of these compounds are trans-anethole and estragole, which are widely used as flavorings in the food and alcoholic beverages industry and have been found in a variety of aromatic species, including basil, fennel, anise and star anise [10]. Currently, essential oils are products of great interest to the cosmetic, pharmaceutical and food industries due to their potential properties that have been characterized as antioxidants, antimicrobials, dyes, and flavorings [11].
2.1. Bioactive Compounds and Their Properties
- In the food industry, important bioactive compounds, such as curcumin (from turmeric, Brazilian saffron), chlorophyll (usually extracted from green leaves), anthocyanins (usually extracted from grape hulls), betanin (from beets), carotenoids (alpha, beta and gamma carotene and lycopene) and xanthophylls, have been found [12].Among these natural pigments, anthocyanins have been well studied for their coloring properties that are responsible for the bright orange, pink, red, violet and blue colors in the flowers and fruits of some plants. They are also known for their antioxidant properties, which play an important role in the prevention of neuronal and cardiovascular diseases, cancer and diabetes [13, 14]. Anthocyanins can be found in the bran and powder of purple wheat, such as cyanidin-3-glucoside, cyanidin-3- (6-malonyl glucoside), cyanidin-3-rutinoside, peonidin-3-glucoside and peonidin-3- (6-malonylglucoside) [15]; in black rice bran (Oryza sativa L.), such as cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, delphinidin, cyanidin, pelargonidin and malvidin, which have been identified as a potential source of dark purple pigment [16]; and in black raspberry (Rubus occidentalis L.), such as cyanidin-3-glucoside, cyanidin-3-sambubioside, cyanidin-3-xylosylrutinoside and cyanidin-rutinoside [17]. Additionally, two anthocyanin groups have been isolated in black currants, such as cyanidins and delphinidins (delphinidin-3-glucoside, delphinidin-3-rutinoside, cyanidin-3-glucoside, cyanidin-3-rutinoside) [18].Carotenoids are responsible for the yellow-orange and red color of food. According to Eggersdorfer and Wyss [19], the main carotenoids found in foods are lutein, β-carotene, zeaxanthin and lycopene. These compounds have properties beneficial to human health due to their antioxidant effects and ability to act individually through other mechanisms; for example, β-carotene has a pro-vitamin A function, while lutein/zeaxanthin is a macular pigment in the eye that benefits ocular health. In addition, they improve cognitive function and cardiovascular health and can help prevent some types of cancer. It is possible to find carotenoids in the fruit of Goji berries (Lycium barbarum L.), mainly the zeaxanthin dipalmitate [20], the peels and the melon seed contain mainly lutein, β-carotene and phenolic compounds [21].On the other hand, aromatic compounds are organic species belonging to different chemical classes, such as alcohols, esters, acids, ketones, aldehydes, terpenes and sulfur compounds [22,23]. These compounds can be found in sources such as rosemary (Rosmarinus officinalis L.) α-pinene, α-fenchone, eucalyptol, trans-sabinene hydrate, camphor, isoborneol and myrtenal [24]; the clove (Syzygium aromaticum) contains eugenol, α-caryophyllene, and 2-methoxy-4-(2-propenyl acetate) phenol [25]. In basil and oregano, α-pinene, limonene, camphor and citronellol (terpenes) can be found [26]. In fennel, two main aromatic compounds, anethole and fenchone, have been identified [27].Food color and aroma are important attributes of quality in the food industries, as they influence the choice and preference of the consumer [28]. The quantity of these compounds is not limited since their functional properties improve the quality and nutritional value of foods. Notably, the use of natural compounds becomes a challenge for industries because of the sensitivity of these substances to pH changes, to heating, to the presence of oxygen and oxidizing agents that can cause their decomposition [29]. Currently, the demand and requirements of consumers for natural foods are constantly growing, and competition among food producers encourages researchers to seek new technological alternatives in terms of the application of these compounds.
3. Supercritical Fluids and Their Application in the Food Industry
- Supercritical fluids are those in which the temperature and pressure are simultaneously a value higher than the critical point, as shown by the shaded region in Figure 1, which is characterized by a density like that of liquids, a diffusivity greater than that of liquids and a very high compressibility higher than that of the ideal gas [30].
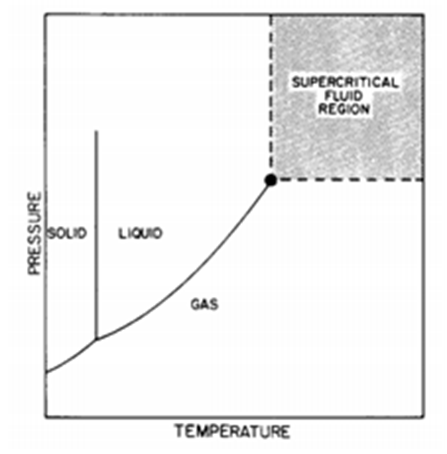 | Figure 1. Phase diagram, adapted from Jean and Debenedetti [30] |
4. Supercritical Precipitation Technique Antisolvent (SAS)
- The supercritical antisolvent process (SAS) is based on the precipitation of the solutes dissolved in conventional liquid solvent (dichloromethane, ethanol, methanol, dimethylsulfoxide, etc.) using a supercritical fluid. The supercritical fluid saturates the liquid solvent, resulting in precipitation of the solute due to the antisolvent effect [34].It is important to keep in mind that this technique is based on two prerequisites: first, the liquid solvent and the anti-solvent (CO2) must be completely miscible under the process conditions, and finally, the solute must be insoluble in the solvent-antisolvent mixture [40]. In the experimental part, the procedure normally begins with the delivery of supercritical CO2 at a constant flow rate to the precipitation chamber until the desired pressure is reached. Then, a pure solvent is sent through an injector to the pressurized chamber to obtain a stable composition condition during the precipitation of the solute. At that point, the flow of the organic solvent stops, and the liquid solution formed by the organic solvent and the solute to be micronized are delivered through the nozzle. Once the fixed amount of organic solution is injected, the pump for liquids is stopped. However, the supercritical CO2 continues to flow to wash the chamber of the residual content of liquid solubilized in the supercritical anti-solvent. If the final purge with pure CO2 is not performed, the solvent condenses during depressurization and can solubilize or modify the dust [40,41]. In Figure 2, general schematization of the procedure is shown.
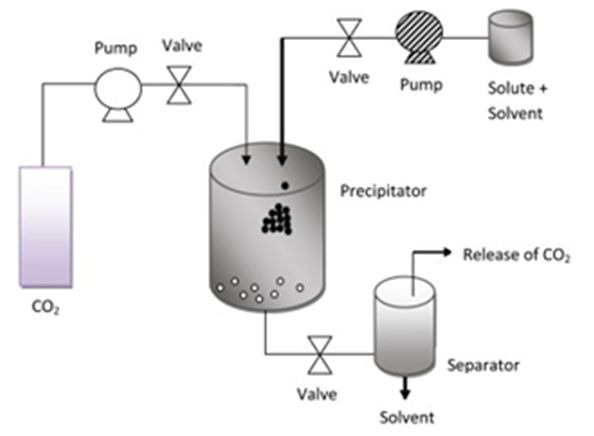 | Figure 2. Schematic process the supercritical antisolvent process, adapted from Fahim, t. k. et al [34] |
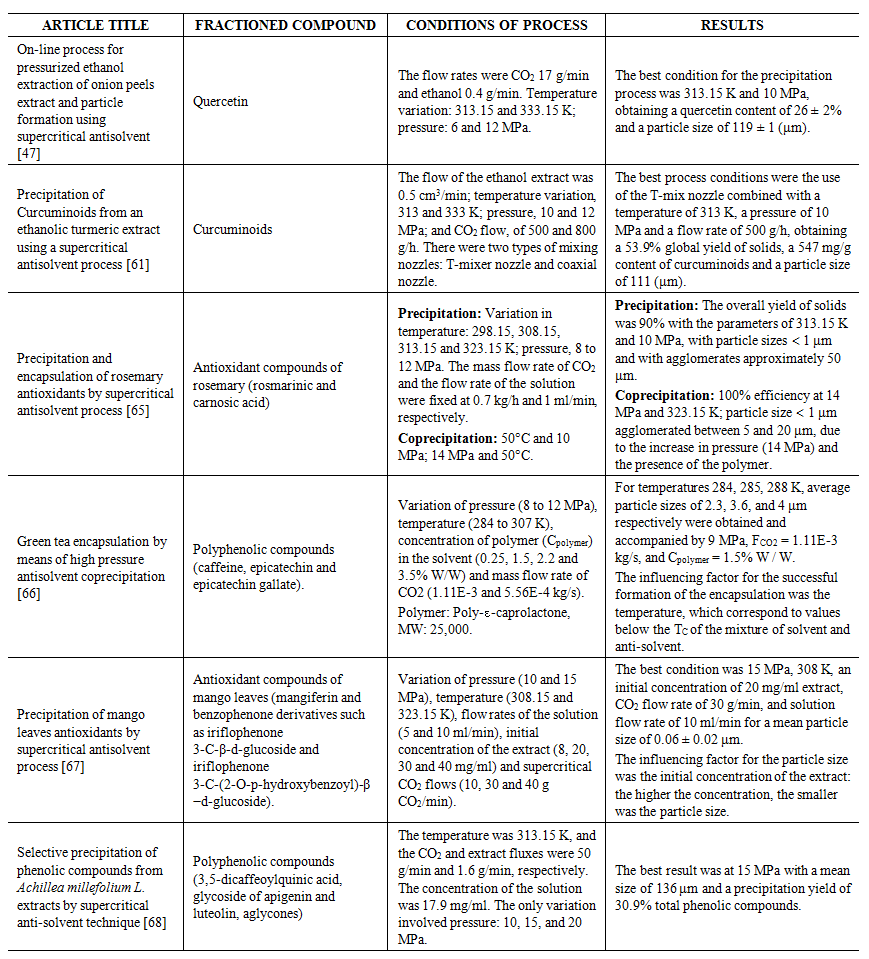 | Table 1. Application of the SAS process in plant extracts |
4.1. Influencing Factors in Particle Formation and Morphology
- Many factors of importance for production or control in the formation and size of the particles were found; several studies have been realized to describe this phenomenon:First is the influencing factor of the solubility of the solute in the mixture of solvent and antisolvent for the formation of amorphous particles or the production of crystals. If the substance to be processed is slightly soluble in the mixture of solvent and antisolvent, the level of supersaturation is moderate, and crystallization is produced. If the solute is slightly soluble in the mixture, the supersaturation level is much higher and the precipitation of amorphous particles occurs; the pressure plays an important role in this behavior, since when increasing the pressure in a system where the solute is slightly soluble in the mixture, the solubility of the solute increases, decreasing the level of supersaturation, and when the solute is poorly soluble in the mixture, then the level of supersaturation increases [54].The gas-liquid phase balance between the solvent and the supercritical CO2 is another important consideration in the SAS process. Campardelli et al. [55] stated that it is important to know the equilibrium of the solvent-CO2 phase and to consider that the solubility behavior of a binary system can change with the addition of a third component. Therefore, it was realized three experiments. First, it was evaluated the dependence of the morphology in the ternary phase of dimethylsulfoxide (DMSO) plus cefonicid (CEF) and CO2, obtaining at 313K the values of the critical points of the mixture of 8.81 MPa; XCO2 = 0.923 and 10.71 MPa; and XCO2 = 0.927 for the binary and ternary systems, respectively. In the second place, the authors performed tests with to values lower than the critical point of the mixture (10.5 MPa) observing expanded microparticles or the formation of two phases in the precipitator. Finally, it was used values higher than the critical point of the mixture (18 Mpa-333K) to the precipitation of the CEF, observing nanoparticles corresponding to conditions visualized by the ternary equilibrium system. Similarly, Cardoso et al. [56] mentioned the importance of the phase behavior of the ternary system where the results of particle size and morphology could be anticipated; however, the absence of information leads to the need to study each particular system (antisolvent, organic solvent and compounds for micronizing) or to preside the behavior through the binary phase diagram that should be performed first to clarify the behavior of the solvent-CO2 and thereby analyze the considerations of fluid dynamics, nucleation or mass transfer to a given ternary system.In the literature, measurements of the phase behavior of binary systems, such as CO2-ethanol, CO2-water, CO2-methanol, and CO2-dichloromethane [57-59] have been reported.The temperature, pressure, and concentration of the liquid solution (solute-solvent) are also important. In a previous study [60], researchers evaluated these three parameters using gadolinium acetate (GDAC) as a model compound and DMSO as a solvent. First, the pressure variation was evaluated from 9 to 20 MPa, keeping the temperature (313 K) and the concentration of the liquid solution of 60 mg/ml constant, observing that with the increase in pressure, a smaller particle size is produced, achieving a transition from microparticles to nanoparticles (0.21 μm at 20 MPa) in the proximity of the critical point of the DMSO-CO2 system mix and in fully developed supercritical conditions. Then, the behavior of the concentration of the liquid solution of 20 to 200 mg/ml was evaluated, keeping the temperature (313 K) and the pressure (15 MPa) constant. For this case, it was observed that an increase in the concentration of GDAC in the liquid solution was accompanied by an increase in the average particle size and the size distribution, where only the nanoparticles were present at concentrations of 20 and 25 mg/ml (0.09 μm) for both cases. Finally, a temperature variation of 308 to 333 K was carried out, maintaining constant values of pressure (15 MPa) and concentration (60 mg/ml) for this evaluation, which revealed only microparticles and extended micro particles, considering that the experiments carried out with higher temperatures show a sequence of reverse precipitation mechanism observed for the increase in pressure.The type of mixer for CO2 fluids and extract (solute-solvent) in the precipitation vessel is influential; the use of a T-mixer causes turbulence between the fluids, generating increases in the mass transfer between the CO2 and the organic solvent because of the rapid diffusion of CO2 into the drop. This phenomenon accelerates supersaturation and nucleation, which results in the formation of smaller particles, and when using a T-connector, the opposite effect occurs [61].A study by De Marco et al. [62] evaluated the effect of soluble solvent mixtures such as ethanol, dimethylsulfoxide and N-methylpyrrolidone with a slightly soluble solvent (acetone) on a polyvinylpyrrolidone biopolymer (PVP). These experiments demonstrated that the use of suitable solvent mixtures is effective for the decrease in the average particle size of PVP and the contraction of the particle size distribution generating the production of nanoparticles with an average diameter of 0.22 μm, 0.16 μm to 0.11 μm. The acetone acts as a modifier of the particle morphology, interpreting the behavior with the relation of "a bad solvent" - "a good solvent", where the polymer tends to reject the acetone molecules, precipitating in a conformation that is more compact [63].Petit-Gas et al. [64] studied the role of hydrodynamics in describing the jet dispersion of pure liquid organic solvents and the influence of the presence of solute, temperature, pressure, feed configuration and variation of the liquid flow rate of 0.1 at 9 ml/min. As a result, for ethanol, the formation of an asymmetric jet on the shaft at 313 K and 11 MPa (jet velocity of 0.09 m / s) was shown. For acetone, there was an asymmetric jet at 308 K and 12 MPa (speed of 0.13 m/s) and an atomized jet for acetone at 308 K and 12 MPa (jet velocity of 0.89 m/s), highlighting that even under conditions of complete miscibility between the binary systems, a boundary between the dispersed and continuous phase was observed because it was not above the value of the transition pressure between the biphasic and monophasic regions. The rates of critical atomization for the systems were between 0.33 and 0.42 m/s for the pure solvents and 0.39-0.41 m/s for the systems in the presence of solute (sulfathiazole). The authors affirm that obtaining closed and low values did not influence the chemical nature of the solvent, the variations in the physicochemical properties of the dispersed phase, the internal diameter of the capillary used (only generating differences in the Reynolds number) or the presence of the solute in the critical atomization rate. After studying the crystallization under conditions like those for analyzing the dispersion of the jet, it was evident that the key factor for the formation of finer particles seems to be the mixture's state more than the state of atomization, even if the characteristics of the powder are more homogeneous when atomized by the jet.
5. Conclusions and Future Expectations
- The SAS processing of natural extracts is an effective way to obtain micro- and nanoparticles, thereby achieving availability, protection, and concentration as well as consequently increasing the quality of natural extracts.Due to the feasibility of using the SAS technique and its excellent results for the micronization and encapsulation of natural compounds, it is necessary to increase research on other bioactive compounds in the food industry. Furthermore, it would also be interesting to achieve relevant applications of this technique in industry.A prerequisite for the micronization or formation of precipitates is the gas-liquid phase diagram of the supercritical fluid and the solvent that will be used as a starting point for the choice of operating parameters. Although good results have been obtained with this criterion, thermodynamic studies involving the presence of two or more solutes in the equilibrium of the phases are needed since this approach could improve the prediction of particle formation and the coprecipitations would be completely successful.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML