| [1] | N. S. Bentsen and I. M. Møller, “Solar energy conserved in biomass: Sustainable bioenergy use and reduction of land use change,” Renew. Sustain. Energy Rev., vol. 71, pp. 954–958, 2017. |
| [2] | R. A. M. Boloy, M. E. Silva, A. E. Valle, J. L. Silveira, and C. E. Tuna, “Thermoeconomic analysis of hydrogen incorporation in a biodiesel plant,” Appl. Therm. Eng., vol. 113, pp. 519–528, 2017. |
| [3] | D. Singh, S. K. Singal, M. O. Garg, P. Maiti, S. Mishra, and P. K. Ghosh, “Transient performance and emission characteristics of a heavy-duty diesel engine fuelled with microalga Chlorella variabilis and Jatropha curcas biodiesels,” Energy Convers. Manag., vol. 106, pp. 892–900, Dec. 2015. |
| [4] | H. Hosseinzadeh-Bandbafha, M. Tabatabaei, M. Aghbashlo, M. Khanali, and A. Demirbas, “A comprehensive review on the environmental impacts of diesel/biodiesel additives,” Energy Convers. Manag., vol. 174, pp. 579–614, Oct. 2018. |
| [5] | G. Aca-Aca, M. I. Loría-Bastarrachea, F. A. Ruiz-Treviño, and M. Aguilar-Vega, “Transesterification of soybean oil by PAAc catalytic membrane: Sorption properties and reactive performance for biodiesel production,” Renew. Energy, vol. 116, pp. 250–257, 2018. |
| [6] | A. Rahimi, G. Moradi, S. Abolhasan Alavi, and M. Ardjmand, “Simultaneous extraction of rapeseed oil and conversion to biodiesel using heterogeneous and homogeneous catalysts,” Environ. Prog. Sustain. Energy, vol. 37, no. 1, 2017. |
| [7] | W. Wu, M. Zhu, and D. Zhang, “An experimental and kinetic study of canola oil transesterification catalyzed by mesoporous alumina supported potassium,” Appl. Catal. A Gen., vol. 530, pp. 166–173, 2017. |
| [8] | N. A. Pannilawithana, H. M. K. K. Pathirana, N. A. Pannilawithana, and H. M. K. K. Pathirana, “A green method to produce a green method to produce biodiesel from palm olein oil,” Journal of Oil Palm Research, vol. 29, no. June, pp. 267–277, 2017. |
| [9] | K. Nitin Uttamrao and S. Premkartikkumar, “Production of biodiesel from refined cotton seed oil as well as its effects as fuel in diesel engine,” International Journal of Mechanical and Production Engineering Research and Development, vol.8, pp. 817-824, 2018. |
| [10] | S. Nitièma-Yefanova, L. Coniglio, R. Schneider, R. H. C. Nébié, and Y. L. Bonzi-Coulibaly, “Ethyl biodiesel production from non-edible oils of Balanites aegyptiaca, Azadirachta indica, and Jatropha curcas seeds - Laboratory scale development,” Renew. Energy, vol. 96, pp. 881–890, 2016. |
| [11] | N. Mohd Kamal, W. A. Wan Abu Bakar, and R. Ali, “Catalytic optimization and physicochemical studies over Zn/Ca/Al2O3catalyst for transesterification of low grade cooking oil,” Energy Convers. Manag., vol. 137, pp. 113–120, 2017. |
| [12] | A. S. Rad, M. H. Nia, F. Ardestani, and H. Nayebzadeh, “Esterification of Waste Chicken Fat: Sulfonated MWCNT Toward Biodiesel Production,” Waste and Biomass Valorization, vol. 9, no. 4, pp. 591–599, Apr. 2018. |
| [13] | B. Q. Araújo, R. C. da R. Nunes, C. V. R. de Moura, E. M. de Moura, A. M. das G. L. Citó, and J. R. dos Santos Júnior, “Synthesis and Characterization of Beef Tallow Biodiesel,” Energy & Fuels, vol. 24, no. 8, pp. 4476–4480, Aug. 2010. |
| [14] | S. P. Singh and D. Singh, “Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review.” Renewable and Sustainable Energy Reviews, vol. 14, pp. 200-2016, 2010. |
| [15] | G. Knothe, “Current perspectives on biodiesel,” INFORM - International News on Fats, Oils and Related Materials, vol. 13, no. 12, pp. 900–903, 2002. |
| [16] | P. Felizardo, M. J. Neiva Correia, I. Raposo, J. F. Mendes, R. Berkemeier, and J. M. Bordado, “Production of biodiesel from waste frying oils,” Waste Manag., vol. 26, no. 5, pp. 487–494, Jan. 2006. |
| [17] | M. R. Avhad and J. M. Marchetti, “Innovation in solid heterogeneous catalysis for the generation of economically viable and ecofriendly biodiesel: A review,” Catal. Rev. - Sci. Eng., vol. 58, no. 2, pp. 157–208, 2016. |
| [18] | J. P. Da Costa Evangelista, A. D. Gondim, L. Di Souza, and A. S. Araujo, “Alumina-supported potassium compounds as heterogeneous catalysts for biodiesel production: A review,” Renew. Sustain. Energy Rev., vol. 59, pp. 887–894, 2016. |
| [19] | B. Freedman, E. H. Pryde, and T. L. Mounts, “Variables affecting the yields of fatty esters from transesterified vegetable oils,” J. Am. Oil Chem. Soc., vol. 61, no. 10, pp. 1638–1643, Oct. 1984. |
| [20] | M. Zabeti, W. M. Ashri, W. Daud, and M. K. Aroua, “Activity of solid catalysts for biodiesel production: A review,” Fuel Process. Technol., vol. 90, pp. 770–777, 2009. |
| [21] | T. F. Dossin, M.-F. Reyniers, R. J. Berger, and G. B. Marin, “Simulation of heterogeneously MgO-catalyzed transesterification for fine-chemical and biodiesel industrial production,” Appl. Catal. B Environ., vol. 67, no. 1–2, pp. 136–148, Sep. 2006. |
| [22] | A. Tiwari, V. M. Rajesh, and S. Yadav, “Biodiesel production in micro-reactors: A review,” Energy Sustain. Dev., vol. 43, pp. 143–161, Apr. 2018. |
| [23] | M. Canakci and J. Van Gerpen, “Biodiesel production via acid catalysis,” Trans. Am. Soc. Agric. Eng., vol. 42, no. 5, pp. 1203–1210, 1999. |
| [24] | A. E. Atabani, A. S. Silitonga, I. A. Badruddin, T. M. I. Mahlia, H. H. Masjuki, and S. Mekhilef, “A comprehensive review on biodiesel as an alternative energy resource and its characteristics,” Renew. Sustain. Energy Rev., vol. 16, no. 4, pp. 2070–2093, May 2012. |
| [25] | Y. M. Sani, W. M. A. W. Daud, and A. R. Abdul Aziz, “Activity of solid acid catalysts for biodiesel production: A critical review,” Appl. Catal. A Gen., vol. 470, pp. 140–161, 2014. |
| [26] | J. Van Gerpen, “Biodiesel processing and production,” Fuel Process. Technol., vol. 86, no. 10, pp. 1097–1107, Jun. 2005. |
| [27] | L. Wu, T. Wei, Z. Tong, Y. Zou, Z. Lin, and J. Sun, “Bentonite-enhanced biodiesel production by NaOH-catalyzed transesterification of soybean oil with methanol,” Fuel Process. Technol., vol. 144, pp. 334–340, Apr. 2016. |
| [28] | Y. M. Sani, W. M. A. W. Daud, and A. R. Abdul Aziz, “Solid acid-catalyzed biodiesel production from microalgal oil—The dual advantage,” J. Environ. Chem. Eng., vol. 1, no. 3, pp. 113–121, Sep. 2013. |
| [29] | A. B. Fadhil, E. T. B. Al-Tikrity, and A. M. Khalaf, “Transesterification of non-edible oils over potassium acetate impregnated CaO solid base catalyst,” Fuel, vol. 234, pp. 81–93, Dec. 2018. |
| [30] | N. Shibasaki-Kitakawa, T. Tsuji, M. Kubo, and T. Yonemoto, “Biodiesel Production from Waste Cooking Oil Using Anion-Exchange Resin as Both Catalyst and Adsorbent,” BioEnergy Res., vol. 4, no. 4, pp. 287–293, Dec. 2011. |
| [31] | A. K. Endalew, Y. Kiros, and R. Zanzi, “Heterogeneous catalysis for biodiesel production from Jatropha curcas oil (JCO),” Energy, vol. 36, no. 5, pp. 2693–2700, May 2011. |
| [32] | M. D. Putra, C. Irawan, Udiantoro, Y. Ristianingsih, and I. F. Nata, “A cleaner process for biodiesel production from waste cooking oil using waste materials as a heterogeneous catalyst and its kinetic study,” J. Clean. Prod., vol. 195, pp. 1249–1258, Sep. 2018. |
| [33] | J. L. Solis, A. L. Berkemar, L. Alejo, and Y. Kiros, “Biodiesel from rapeseed oil (Brassica napus) by supported Li2O and MgO,” Int. J. Energy Environ. Eng., vol. 8, no. 1, pp. 9–23, 2017. |
| [34] | W. Ye, G. Yujie, D. Hui, L. Mingchao, L. Shejiang, H. Xu., “Kinetics of transesterification of palm oil under conventional heating and microwave irradiation, using CaO as heterogeneous catalyst,” Fuel, vol. 180, pp. 574–579, 2016. |
| [35] | N. Nurhayati, S. Anita, T. A. Amri, and A. Linggawati, “Esterification of Crude Palm Oil Using H2SO4 and Transesterification Using CaO Catalyst Derived from Anadara granosa,” Indones. J. Chem., vol. 17, no. 2, p. 309, 2017. |
| [36] | H. Li, S. Niu, C. Lu, and J. Li, “Calcium oxide functionalized with strontium as heterogeneous transesterification catalyst for biodiesel production,” Fuel, vol. 176, pp. 63–71, 2016. |
| [37] | M. B. Navas, I. D. Lick, P. A. Bolla, M. L. Casella, and J. F. Ruggera, “Transesterification of soybean and castor oil with methanol and butanol using heterogeneous basic catalysts to obtain biodiesel,” Chem. Eng. Sci., vol. 187, pp. 444–454, 2018. |
| [38] | F. R. Panjaitan, S. Yamanaka, and Y. Kuga, “Soybean oil methanolysis over scallop shell-derived CaO prepared via methanol-assisted dry nano-grinding,” Adv. Powder Technol., vol. 28, no. 7, pp. 1627–1635, 2017. |
| [39] | F. P. De Sousa, G. P. Dos Reis, C. C. Cardoso, W. N. Mussel, and V. M. D. Pasa, “Performance of CaO from different sources as a catalyst precursor in soybean oil transesterification: Kinetics and leaching evaluation,” J. Environ. Chem. Eng., vol. 4, no. 2, pp. 1970–1977, 2016. |
| [40] | M. R. Avhad et al., “Enhancing Biodiesel Production Using Green Glycerol-Enriched Calcium Oxide Catalyst: An Optimization Study,” Catal. Letters, vol. 148, no. 4, pp. 1169–1180, 2018. |
| [41] | G. P. Benedictto, R. M. Sotelo, B. O. Dalla Costa, G. Fetter, and E. I. Basaldella, “Potassium-containing hydroxylated hydrotalcite as efficient catalyst for the transesterification of sunflower oil,” J. Mater. Sci., vol. 53, no. 18, pp. 12828–12836, Sep. 2018. |
| [42] | Y. Ma, Q. Wang, L. Zheng, Z. Gao, Q. Wang, and Y. Ma, “Mixed methanol/ethanol on transesterification of waste cooking oil using Mg/Al hydrotalcite catalyst,” Energy, vol. 107, pp. 523–531, 2016. |
| [43] | H. Brasil et al., “Preparation of Hydrotalcite–Hydroxyapatite Material and Its Catalytic Activity for Transesterification of Soybean Oil,” Catal. Letters, vol. 147, no. 2, pp. 391–399, 2017. |
| [44] | E. Rodrigues, H. Brasil, T. Barros, C. Pereira, O. Almeida, and U. E. De Campinas, “Synthesis and characterization of carbon nanotube doped hydrotalcite-hydroxyapatite material and its application in the transesterification reaction catalysis,” vol. 64, pp. 166–175, 2018 (in portuguese). |
| [45] | M. Banchero and G. Gozzelino, “A Simple Pseudo-Homogeneous Reversible Kinetic Model for the Esterification of Different Fatty Acids with Methanol in the Presence of Amberlyst-15,” Energies, vol. 11, no. 7, p. 1843, 2018. |
| [46] | M. Kuzminska, R. Backov, and E. M. Gaigneaux, “Behavior of cation-exchange resins employed as heterogeneous catalysts for esterification of oleic acid with trimethylolpropane,” Appl. Catal. A Gen., vol. 504, pp. 11–16, 2015. |
| [47] | K. F. Haigh, S. Z. Abidin, G. T. Vladisavljević, and B. Saha, “Comparison of Novozyme 435 and Purolite D5081 as heterogeneous catalysts for the pretreatment of used cooking oil for biodiesel production,” Fuel, vol. 111, pp. 186–193, 2013. |
| [48] | Y. Jamal, A. Rabie, and B. O. Boulanger, “Determination of methanolysis rate constants for low and high fatty acid oils using heterogeneous surface reaction kinetic models,” React. Kinet. Mech. Catal., vol. 114, no. 1, pp. 63–74, 2015. |
| [49] | S. Z. Abidin, K. F. Haigh, and B. Saha, “Esterification of free fatty acids in used cooking oil using ion-exchange resins as catalysts: An efficient pretreatment method for biodiesel feedstock,” Ind. Eng. Chem. Res., vol. 51, no. 45, pp. 14653–14664, 2012. |
| [50] | G. Paterson, T. Issariyakul, C. Baroi, A. Bassi, and A. Dalai, “Ion-exchange resins as catalysts in transesterification of triolein,” Catalysis Today, vol. 212, pp. 157-163, 2013. |
| [51] | K. L. T. Rodrigues, V. M. D. Pasa, and É. C. Cren, “Kinetic modeling of catalytic esterification of non-edible macauba pulp oil using macroporous cation exchange resin,” J. Environ. Chem. Eng., vol. 6, no. 4, pp. 4531–4537, 2018. |
| [52] | B. M. E. Russbueldt and W. F. Hoelderich, “New sulfonic acid ion-exchange resins for the preesterification of different oils and fats with high content of free fatty acids,” Appl. Catal. A Gen., vol. 362, no. 1–2, pp. 47–57, 2009. |
| [53] | N. Shibasaki-Kitakawa, K. Hiromori, T. Ihara, K. Nakashima, and T. Yonemoto, “Production of high quality biodiesel from waste acid oil obtained during edible oil refining using ion-exchange resin catalysts,” Fuel, vol. 139, pp. 11–17, 2015. |
| [54] | A. Martínez, G. E. Mijangos, I. C. Romero-Ibarra, R. Hernández-Altamirano, V. Y. Mena-Cervantes, and S. Gutiérrez, “A novel green one-pot synthesis of biodiesel from Ricinus communis seeds by basic heterogeneous catalysis,” J. Clean. Prod., vol. 196, pp. 340–349, 2018. |
| [55] | L. Wan, H. Liu, S. Nasreen, I. Lukic, and D. Skala, “High temperature transesterification of soybean oil with methanol using manganese carbonate as catalyst,” Chem. Ind. Chem. Eng. Q., vol. 24, no. 1, pp. 9–22, 2018. |
| [56] | D. Celante, J. V. D. Schenkel, and F. de Castilhos, “Biodiesel production from soybean oil and dimethyl carbonate catalyzed by potassium methoxide,” Fuel, vol. 212, no. September 2017, pp. 101–107, 2018. |
| [57] | A. M. Gonçalves, R. A. B. Lima-Corrêa, J. M. Assaf, and A. R. A. Nogueira, “Lithium and calcium based perovskite type oxides for ethylic transesterification,” Catal. Today, vol. 279, pp. 177–186, 2017. |
| [58] | R. G. Prado et al., “Ethanolysis and Methanolysis of Soybean and Macauba Oils Catalyzed by Mixed Oxide Ca-Al from Hydrocalumite for Biodiesel Production,” Energy and Fuels, vol. 30, no. 8, pp. 6662–6670, 2016. |
| [59] | M. Souza, S. Kuhnen, D. Cristina, C. Kurtz, T. Trapp, and V. Müller, “Óxidos metálicos derivados de materiais tipo hidrotalcitas contendo Ga3+ como catalisadores para síntese de biodiesel etílico,” vol. 40, no. 9, pp. 1074–1081, 2017. |
| [60] | D. Meloni, D. Perra, R. Monaci, M. G. Cutrufello, E. Rombi, and I. Ferino, “Transesterification of Jatropha curcas oil and soybean oil on Al-SBA-15 catalysts,” Appl. Catal. B Environ., vol. 184, pp. 163–173, 2016. |
| [61] | O. Guzhan ˙ Ilgen, A. Ay¸se, N. Akin, and N. Boz, “Investigation of Biodiesel Production from Canola Oil Using Amberlyst-26 as a Catalyst,” Turk J Chem, vol. 33, pp. 289–294, 2008. |
| [62] | Y. Ren et al., “Continuous biodiesel production in a fixed bed reactor packed with anion-exchange resin as heterogeneous catalyst,” Bioresour. Technol., vol. 113, pp. 19–22, Jun. 2012. |
| [63] | N. Shibasaki-Kitakawa, H. Honda, H. Kuribayashi, T. Toda, T. Fukumura, and T. Yonemoto, “Biodiesel production using anionic ion-exchange resin as heterogeneous catalyst,” Bioresour. Technol., vol. 98, no. 2, pp. 416–421, Jan. 2007. |
| [64] | B. Vafakish and M. Barari, “Biodiesel Production by Transesterification of Tallow Fat Using Heterogeneous Catalysis,” Kem. u Ind., vol. 66, no. 1–2, pp. 47–52, 2017. |
| [65] | T. M. Deboni, G. A. M. Hirata, G. G. Shimamoto, M. Tubino, and A. J. de A. Meirelles, “Deacidification and ethyl biodiesel production from acid soybean oil using a strong anion exchange resin,” Chem. Eng. J., vol. 333, no. September 2017, pp. 686–696, 2018. |
| [66] | I. and M. L. M.M. Nasef, Z. Ujang, Ion Exchange Technology I: Theory and Materials. New York, 2012. |
| [67] | L. Ma, Y. Han, K. Sun, J. Lu, and J. Ding, “Kinetic and thermodynamic studies of the esterification of acidified oil catalyzed by sulfonated cation exchange resin,” J. Energy Chem., vol. 24, no. 4, pp. 456–462, 2015. |
| [68] | L. A. Rios, P. P. Weckes, H. Schuster, and W. F. Hoelderich, “Resin catalyzed alcoholysis of epoxidized fatty esters: Effect of the alcohol and the resin structures,” Appl. Catal. A Gen., vol. 284, no. 1–2, pp. 155–161, 2005. |
| [69] | I. Fatimah, D. Rubiyanto, and J. Nugraha, “Preparation, characterization, and modelling activity of potassium flouride modified hydrotalcite for microwave assisted biodiesel conversion,” Sustain. Chem. Pharm., vol. 8, pp. 63–70, Jun. 2018. |
| [70] | M. S. Dhawan and G. D. Yadav, “Insight into a catalytic process for simultaneous production of biodiesel and glycerol carbonate from triglycerides,” Catal. Today, vol. 309, pp. 161–171, Jul. 2018. |
| [71] | S. Sedaghat-Hoor and M. Anbia, “ZnO impregnated MgAl(O) catalyst with improved properties for biodiesel production: The influence of synthesis method on stability and reusability,” Part. Sci. Technol., pp. 1–7, May 2018. |
| [72] | L. Costarrosa et al., “Optimization of the Transesterification of Waste Cooking Oil with Mg-Al Hydrotalcite Using Response Surface Methodology,” Energies, vol. 11, no. 2, p. 302, Jan. 2018. |
| [73] | P. Salinas Hernández, F. Morales Anzures, R. Pérez Hernández, F. Tzompzntzi Morales, and M. A. Romero Romo, “Methanolysis of Simarouba Glauca DC oil with hydrotalcite-type ZnCuAl catalysts,” Catal. Today, Jun. 2018. |
| [74] | A. Navajas et al., “Outstanding performance of rehydrated Mg-Al hydrotalcites as heterogeneous methanolysis catalysts for the synthesis of biodiesel,” Fuel, vol. 211, pp. 173–181, Jan. 2018. |
| [75] | N. D. L. C. Om Tapanes, D. A. Gomes Aranda, J. W. de Mesquita Carneiro, R. S. Perez, and K. de Carvalho Teixeira, “Mg–Al Hydrotalcite as Heterogeneous Catalyst for Transesterification of Jatropha Curcas Oil: Theoretical and Experimental Analysis,” Lett. Org. Chem., vol. 14, no. 7, Jul. 2017. |
| [76] | M. Eskandari, A. Kia, S. Afrasiabi, A. Dara, M. Fahimizadeh, and H. Maddah, “Experimental study of biodiesel fuel production from Euphorbiaceae using a Ca-Al-CO 3 hydrotalcite catalyst,” Energy Sources, Part A Recover. Util. Environ. Eff., vol. 39, no. 2, pp. 225–231, Jan. 2017. |
| [77] | A. Kapil, K. Wilson, A. F. Lee, and J. Sadhukhan, “Kinetic Modeling Studies of Heterogeneously Catalyzed Biodiesel Synthesis Reactions,” Ind. Eng. Chem. Res., vol. 50, no. 9, pp. 4818–4830, May 2011. |
| [78] | F. Cavani, F. Trifirò, and A. Vaccari, “Hydrotalcite-type anionic clays: Preparation, properties and applications.,” Catal. Today, vol. 11, no. 2, pp. 173–301, Dec. 1991. |
| [79] | T. H. Dang, B.-H. Chen, and D.-J. Lee, “Application of kaolin-based catalysts in biodiesel production via transesterification of vegetable oils in excess methanol,” Bioresour. Technol., vol. 145, pp. 175–181, Oct. 2013. |
| [80] | T. M. Deboni, E. A. C. Batista, and A. J. A. Meirelles, “Equilibrium, Kinetics, and Thermodynamics of Soybean Oil Deacidification Using a Strong Anion Exchange Resin,” Ind. Eng. Chem. Res., vol. 54, no. 44, pp. 11167–11179, Nov. 2015. |
| [81] | A. Bouaid, M. Martinez, and J. Aracil, “A comparative study of the production of ethyl esters from vegetable oils as a biodiesel fuel optimization by factorial design,” Chem. Eng. J., vol. 134, no. 1–3, pp. 93–99, 2007. |



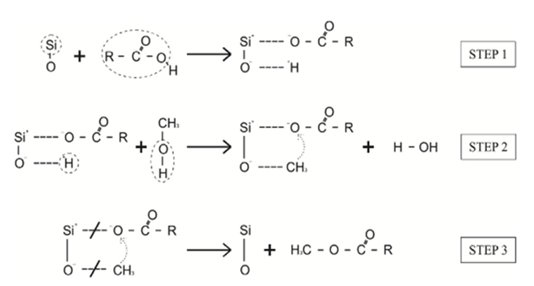
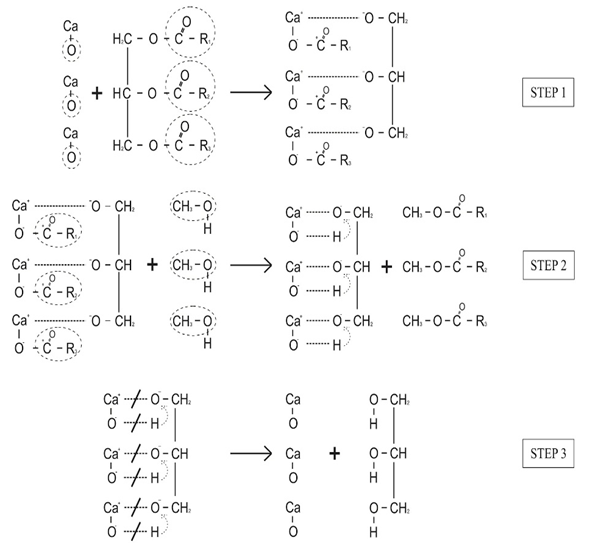
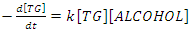
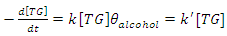
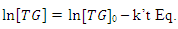
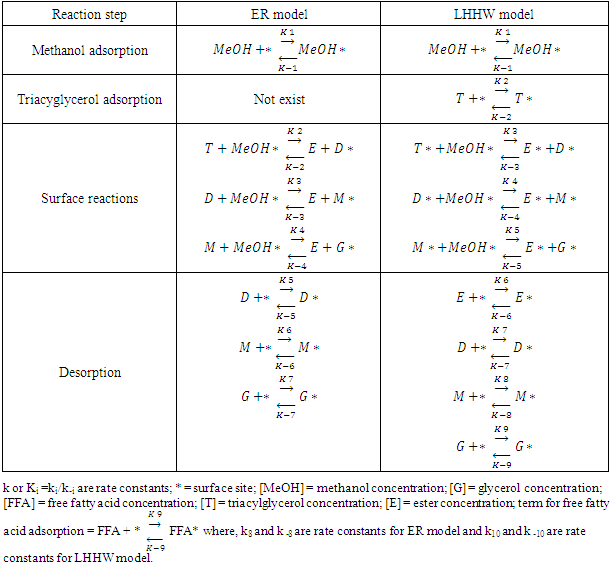
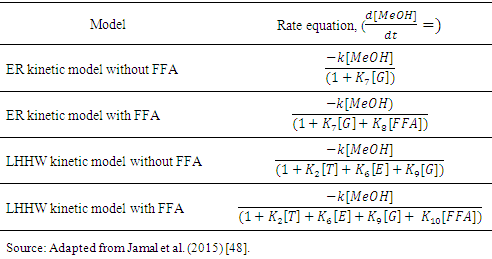
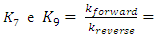 constant of the reaction rate for glycerol; K8 and K10 = rate constant for FFA adsorption; K2= rate constant for triacylglycerol adsorption; K6= constant of the reaction rate for methyl ester; [G] = concentration of glycerol; [FFA] = concentration of FFA; [T] = concentration of triacylglycerol; and [E] = concentration of methyl ester. Jamal et al. (2015) [48] evaluated the methanol consumption over the course of time for each model obtained. Using ER model, the methanol consumption rate constant was 7.48 x 10-4 h-1 without the presence of FFA and 1.94 h-1 with FFA present. For the LHHW model, the constant found for alcohol consumption was 6.20 x 10-2 h-1 without FFA and 1.71 h-1 with FFA. Therefore, the author concluded that the FFA when present increases the rate for the consumption of methanol. The authors also concluded that the mechanism of reaction tends towards a hypothetical ER mechanism due to the methanol in excess used in the reaction. They further stated that the triacylglycerol concentration of the LHHW model provides a small influence in the denominator of the equation at excess level of methanol. But, as the molar ratio of methanol to triacylglycerol reduce, the importance of sorption of triacylglycerol in the resin increases, favoring the model. Thus, informed the reaction parameters, the models used point that methanol adsorption in the catalytic it's the crucial step in reactions where methanol is present in amounts above reaction stoichiometry.Solis et al. (2017) [33] also performed kinetic studies using a catalyst that promoted the highest yield in biodiesel. Mayenite impregnated with 10% lithium (M2) was used to transesterify rapeseed oil. The molar ratio of methanol:oil was 6:1, with a percentage of catalyst of 5% with respect to the mass of oil, subjected to 60°C and stirring of 180 rpm for 2 hours. These parameters were sufficient to reach the maximum yield in FAME conversion (100%).Initially, to model the kinetics of reaction, Solis et al. (2017) [33] made a mass balance on a discontinuous reactor with agitation, according to the equation that follows.
constant of the reaction rate for glycerol; K8 and K10 = rate constant for FFA adsorption; K2= rate constant for triacylglycerol adsorption; K6= constant of the reaction rate for methyl ester; [G] = concentration of glycerol; [FFA] = concentration of FFA; [T] = concentration of triacylglycerol; and [E] = concentration of methyl ester. Jamal et al. (2015) [48] evaluated the methanol consumption over the course of time for each model obtained. Using ER model, the methanol consumption rate constant was 7.48 x 10-4 h-1 without the presence of FFA and 1.94 h-1 with FFA present. For the LHHW model, the constant found for alcohol consumption was 6.20 x 10-2 h-1 without FFA and 1.71 h-1 with FFA. Therefore, the author concluded that the FFA when present increases the rate for the consumption of methanol. The authors also concluded that the mechanism of reaction tends towards a hypothetical ER mechanism due to the methanol in excess used in the reaction. They further stated that the triacylglycerol concentration of the LHHW model provides a small influence in the denominator of the equation at excess level of methanol. But, as the molar ratio of methanol to triacylglycerol reduce, the importance of sorption of triacylglycerol in the resin increases, favoring the model. Thus, informed the reaction parameters, the models used point that methanol adsorption in the catalytic it's the crucial step in reactions where methanol is present in amounts above reaction stoichiometry.Solis et al. (2017) [33] also performed kinetic studies using a catalyst that promoted the highest yield in biodiesel. Mayenite impregnated with 10% lithium (M2) was used to transesterify rapeseed oil. The molar ratio of methanol:oil was 6:1, with a percentage of catalyst of 5% with respect to the mass of oil, subjected to 60°C and stirring of 180 rpm for 2 hours. These parameters were sufficient to reach the maximum yield in FAME conversion (100%).Initially, to model the kinetics of reaction, Solis et al. (2017) [33] made a mass balance on a discontinuous reactor with agitation, according to the equation that follows.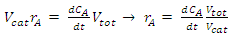
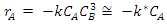
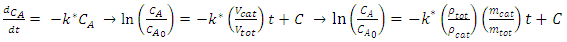
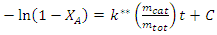
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML