-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2019; 9(4): 103-110
doi:10.5923/j.fph.20190904.01

Effect of Avocado Pear Inclusion on Nutrient Composition and Sensory Qualities of a Complementary Food
B. K. Adeoye, N. O. Philips, I. F. Ani, E. O. Ngozi, N. C. Ajuzie, A. R. Akinlade
Department of Nutrition and Dietetics, Babcock University, Ikeja Lagos, Nigeria
Correspondence to: B. K. Adeoye, Department of Nutrition and Dietetics, Babcock University, Ikeja Lagos, Nigeria.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study determined effect of avocado pear inclusion on the quality of complementary food made from sorghum and soybean using roasting method. Sorghum, soybean and avocado pear were all processed into powder separately and three blends were produced from them. The blends were; sample A (control) which contained 60% sorghum and 40% soybean), sample B (sorghum 60%, soybean 35%, avocado pear 5%) and sample C (sorghum 60%, soybean 30%, avocado pear 10%). The functional properties, nutrients composition and anti-nutritional contents of the samples were determined. The sensory attributes were determined using 9-point hedonic scale and the results were analyzed using analysis of variance (ANOVA) at P<0.05 and means were separated by Duncan multiple range tests. There was no significant difference in the bulk density while the swelling capacity of the samples was significantly different and it increased from the control to the sample with 10% avocado pear. Carbohydrate (60.11 - 55.15 %), protein (20.3 - 20.05 %), moisture (2.40 - 2.12 %), fiber content (7.28 - 6.38 %) of the samples reduced as avocado pear content increased. While the fat (7.80 - 13.80 %), ash (2.10 - 2.50 %), energy (391.88 - 426.60 kcal) increased with the addition of avocado pear. The mineral content (magnesium, iron, potassium, and calcium), phytate and oxalate increased while hydrogen cyanide decreased with increased avocado pear content. There was no significant difference in the acceptability of the samples at the concentration of avocado powder used. Avocado pear inclusion in complementary food can help in the reduction of malnutrition due to its contribution to caloric and micronutrient requirements of complementary food.
Keywords: Avocado pear, Sorghum, Soybean, Complementary food, Quality
Cite this paper: B. K. Adeoye, N. O. Philips, I. F. Ani, E. O. Ngozi, N. C. Ajuzie, A. R. Akinlade, Effect of Avocado Pear Inclusion on Nutrient Composition and Sensory Qualities of a Complementary Food, Food and Public Health, Vol. 9 No. 4, 2019, pp. 103-110. doi: 10.5923/j.fph.20190904.01.
Article Outline
1. Introduction
- Infancy is a time of rapid physical growth as well as physiological, immunological and mental development. During the first year of life, nutritional requirements are at their highest and the time frame between 6 to 24 months is a critical period of growth during which nutrient deficiencies and illness contribute globally to higher rates of under nutrition among children which are under five years of age. In the first four to six months of life the infant’s nutritional requirements can be totally satisfied by breast milk, afterwards complementary foods need to be introduced to amplify energy and nutrient intake [1]. Complementary foods are either liquid or semi-solid given to a child while still breast feeding to ensure optimal physical and mental development [2]. However, infant malnutrition due to poor quality of complementary food is still prominent in developing countries and it underlies more than one-third of child mortality in Nigeria [3]. This could be because commercially made complementary foods are too pricey for low income families which results in these families depending on local staple foods. However, the traditional complementary is not nutrient dense and as such, might not have the nutrients needed by the child in the required amount and relying on it alone leads to under nutrition [4]. This makes protein-energy malnutrition (PEM) to often be associated with traditional complementary foods, when taken with little or no variation [5]. In most West African countries, the first solid and popular complementary foods are based on thin cereal and low in foods from meat, eggs or fish especially among low-income groups due to socioeconomic factors, taboos and ignorance. In Nigeria for instance, such foods are made from maize (Zea mays), millet (Sorghum bicolour), or guinea corn. After successful introduction of cereal gruel, other staple foods in the family menu, such as yam (Dioscorea spp), rice (Oryza sativa), and cocoyam (Colocasia esculenta) are given to the child being mashed, thinned or prechewed. Legumes are rarely used and are introduced much later because of the problems of indigestibility, flatulence and diarrhea associated with their use [6,7].From sixth month onward, complementary foods should be of variety, and balanced mixtures of food items containing cereals, tubers, foods of animal and vegetable origin, and fat should be offered. Only a varied diet guarantees the supply of micronutrients, enhances good eating habits, and prevents the development of anorexia caused by monotonous foods [8-10].Avocado pear is scientifically known as Persea americana. It is native to Mexico and Central America, The world avocado producers are Mexico, Chile, and Sri Lanka [11]. It is rich in mono-unsaturated fats, and has a smooth and creamy texture with unique nutrition profile. Avocado is a low sugar fruit with high fiber and vitamins A, B, C and E. It is an outstanding source of potassium and phosphorus, and contains mono-unsaturated fatty acids which effectively reduces the levels of low density lipoproteins, in the blood helping in the inhibition of coronary heart diseases. The avocado oil from flesh has a digestibility coefficient of 93.8%, which is mainly used as salad oil. Avocado contains a lot of fibers and is rich in vitamins and minerals such as B-vitamins, vitamin K, potassium, copper, vitamin E. Avocados are high in antioxidants including lutein and zeaxanthanin which are important for lowering the risk of different diseases [12].
2. Materials and Methods
2.1. Collection of Raw Materials
- Sorghum (Sorghum bicolour), soybean (Glycine max) and avocado pear (Persea americana) used in making the complementary food were purchased from nearby market (Ilishan-Remo market, Ogun State), while the equipment used in production were provided by the Department of Nutrition and Dietetics, Babcock University.
2.2. Sorghum Preparation
- Method of Nwakalor and Obi [13] was adopted with little modification. Sorghum was sorted by removing unwanted materials and washing thrice with clean water in a bowl. The grain was then dried at 100°C for 4 hours and roasted at 170°C in a hot air oven until a slight brown colouration was obtained. The sorghum was then milled into fine flour of 0.5 – 1.0 mm particle size and packaged in an air-tight container (Fig. 1).
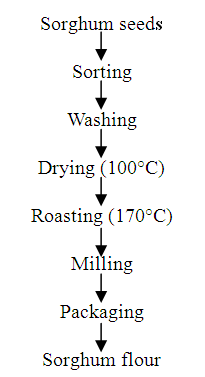 | Figure 1. Modified method of sorghum processing |
2.3. Soybean Preparation
- Fig. 2 presents the processing of soybean into flour. The soybean was sorted to remove shafts and other unwanted materials, it was then soaked for 6 hours and dehulled manually before been dried to a constant weight using the hot air oven at 100°C. The dried seeds were roasted at a temperature of 170°C until a characteristic slight brown colouration was obtained. The seeds were then cooled and grinded into fine flour of 0.5 – 1 mm particle size [14].
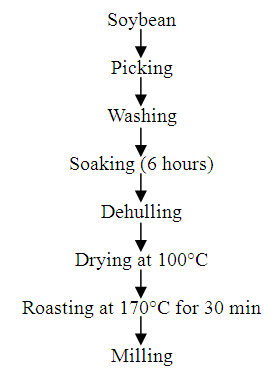 | Figure 2. Soybean processing |
2.4. Avocado Powder Preparation
- Avocado pear in its early ripening stage was used. It was washed, peeled and then sliced extremely thin. These slices were then placed in pans and placed in a hot air oven at 80°C and were periodically removed from the oven to turn over. After drying, a knife was used to scrape the dried avocado from the pan and the dried avocado was then milled into powder and placed in an air-tight container (Fig. 3).
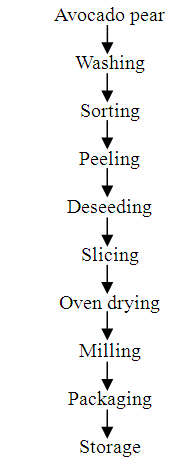 | Figure 3. Processing of avocado pear powder |
2.5. Formulation of the Complementary Food Samples
- The grinded sorghum, soybean and avocado pear were combined differently for formulation of samples A, B and C as follows;

2.6. Functional Properties
2.6.1. Bulk Density
- This was determined using the procedure described by Onwuka [15]. Ten grams of each sample was weighed and carefully filled into the measuring cylinder (100 mL capacity). The bottom of the cylinder was gently tapped on the laboratory bench several times until there was no further reduction of the sample level. The bulk density was calculated thus;
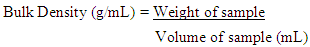
2.6.2. Water Swelling Capacity
- The swelling power of each sample was calculated by the method of Ukpabi and Ndimele [16]. Five gram of each flour sample was weighed into clean, dry graduated (50 mL) cylinder. The flour samples were mildly leveled into it and the volume noted. Distilled water (10 mL) was added to each sample. The cylinder was swirled and allowed to stand for 60 minutes while the change in volume (swelling) was recorded. The swelling index of the sample was given by the formula;
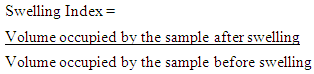
2.7. Nutrient Composition
2.7.1. Moisture Content
- Moisture was determined by the method described by Association of Official Analytical Chemist [17]. A clean empty and dry crucible was placed on an analytical balance, weighed and the weight was taken. Five gram of samples was added to the crucible and the total weight was taken before been placed in the oven for 4 hours at 104 degree centigrade. The crucible was removed after 4 hours and was placed in a desiccator to cool before been weighed.

2.7.2. Protein Content
- Nitrogen in the samples was detected by the routine semi-micro Kjeldahl procedure [17]. This consists of three stages of analysis namely; Digestion, Distillation and Titration. 0.5 g of each finely grinded dried sample was weighed carefully into the Kjeldahl digestion tubes. One Kjeldahl catalyst tablet was added to each of the tubes followed by addition of 12 to 15 ml of conc. H2SO4. These were then set in the appropriate hole of the Digestion Block Heaters in a fume cupboard. The digestion was left for 1 hour and 15 minutes after which a clear colourless solution remained and the digest was left in the fume cupboard to cool and was diluted with some quantities of distilled water to lower the concentration of the acid. The green colour solution obtained was then titrated against 0.1 N H2SO4 contained in a 50 ml burette. At the end point the green colour turned to wine colour which indicated that all the Nitrogen trapped as Ammonium Borate [(NH4)2BO3] have been removed as Ammonium Chloride [(NH4)2SO4].
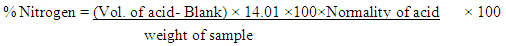 % Protein= value of Nitrogen × 6.25 (conversion factor)
% Protein= value of Nitrogen × 6.25 (conversion factor)2.7.3. Fat Content
- Fat content was determined using the method of AOAC [17]. One gram of the sample was weighed and placed in a thimble (a white paper cup for fat extraction), covered with a defatted cotton wool and labeled. It was then placed into an oven for 1 hour to dry further and later placed in a desiccator and the weight was taken and recorded. 50 ml of petroleum ether was added into the cup and the thimbles and cups were placed into the fat extraction machine and extracted for 11/2 hours. The cups were removed and further dried in the oven for 15 min before been placed in a desiccator and weighing.
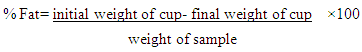
2.7.4. Ash Content
- A clean, empty and dry crucible was placed on an analytical balance and 2g of the sample was added to the crucible, it was kept for 7 hours in the furnace, the crucible was then removed to the desiccator for cooling and then weighed while warm [17].
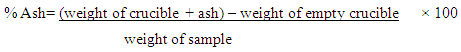
2.7.5. Fiber Content
- The fiber cap was weighed and recorded, 1 g of the sample was poured into the fiber cap and dipped into a solution of dilute H2SO4 (1.25%). It was placed on a hot plate and left to digest for 35 min after which it was removed and washed in distilled water. The procedure was repeated by placing the fiber cap into 1.25% KOH for another 35 minutes. It was removed and rinsed with hot water and then placed into the oven for 3 to 4 hours to dry. An empty crucible was weighed and recorded and the weight of the fiber cap and dry sample. The dry cap was placed into the heated furnace for 1 hour and later into the desiccator for cooling before been weighed [17]. Fiber content of the samples was appropriately calculated.
2.7.6. Total Carbohydrate Content
- Carbohydrate content was determined using the method of AOAC [17] by subtracting the total sum of the percentage of fat, moisture, ash, crude fiber and protein content from one hundred (100).100- (Values of Crude Protein + Fat+ Crude Fiber + Ash + Moisture)
2.7.7. Energy Content
- Energy content was determined using the method of Atwater and Woods [18]. The food total energy was estimated using (4 × proteins + 4× carbohydrate + 9×fat) as the energy values are 17 KJ/g (4.0 kcal/g) for protein, 37 KJ/g (9.0 kcal/g) for fat and 17 KJ/g (4.0 kcal/g) for carbohydrate.
2.7.8. Mineral Analysis
- AOAC methods [17] were used to determine the mineral content of the samples. 1gram of each sample was digested with nitric, perchloric acid and sulphuric acid in a ratio of 9:2:1 respectively. The mixture was filtered and the filtrate was poured into a 5 ml volumetric flask which was loaded to Atomic Absorption Spectrophotometer. The standard curve for each mineral (calcium, magnesium, iron, phosphorus) was prepared from known standards and the mineral value of samples estimated against that of a standard curve.
2.8. Anti-nutritional Factors
2.8.1. Phytate
- Procedure for Phytate determination was described by Lucas and Markakes [19]. One gram of the powdered sample was measured into a conical flask after which 30 ml of 2% HCL was added and soaked for 3 hours before been filtered. 25 ml of the filtrate was pippeted into a conical flask and 5 ml of 0.3% Ammonium thiocyanate was added. 53.5 ml of distilled H2O was added and the solution was titrated against 0.005 M ferric chloride (FeCl3) solution until a reddish brown colour persist for 5 minutes. The phytic acid value was calculated using the formula;(titre value ×0.00195×1.19×100)
2.8.2. Oxalate
- The alkaloid was determined using a method prescribed by Harborne [20]. 1 g of the sample was weighed and 75 ml of 1.5N H2SO4 was added to the sample in a 100 ml conical flask and stirred for 1hour using a magnetic stirrer and then filtered using Watman filter paper. Twenty-five milliliter of the extract was pipetted into another conical flask and 25 ml of the extract was titrated when hot against 0.l N KMnO4 solution to a faint pink colour point and the titre value was recorded.Oxalate = (Titre value x 0.9004) mg/g
2.8.3. Hydrogen Cyanide
- The procedure used was that of Anhwange [21].Ten grams of each of the ground samples were soaked in the mixture of 200 cm3 of distilled water and 10 cm3 of orthophosphoric acid. The mixture was kept for 12 hours to release all the bonded cyanide. The mixture was then distilled until 150 cm3 of the distillate was collected. 20 cm3 of the distillate was added to a conical flask containing 40 cm3 of distilled water, 8 cm3 of ammonia solution and 2 cm3 of potassium iodide (5 %) solution. The mixture was titrated with silver nitrate to faint but permanent turbidity. Replicates determination were done for each of the samples.
2.9. Sensory Evaluation
- Gruel of the different blends was prepared by mixing 100 g of each the samples with 200 ml of water and an additional 240 ml was added to each sample during cooking which lasted for ten minutes.Sensory evaluation was conducted on the complementary food samples and ten panelists comprising of female students in Babcock University participated in the study. The panelists were served with similar quantity of samples placed in white plates and water was provided for rinsing in-between evaluation of the samples to avoid being biased. The attributes that were assessed are colour, texture, taste, aroma and general acceptability [22].
2.10. Statistical Analysis
- One way analysis of variance (ANOVA) and Duncan Multiple Range Tests were used to determine the significant difference and separate means using Statistical Package for Social Sciences (SPSS) version 20.0.
3. Results
- Plate 1 presented the dried avocado pear before been grounded to powder.
 | Plate 1. Dried avocado pear |
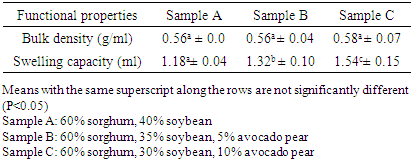
3.1. Functional Properties
- The results of the functional properties of the complementary diet are presented in Table 1. The bulk density increased from the control sample A (0.56 g/ml) to sample C (0.58 g/ml), but not significantly. The water swelling capacity also increased significantly with the addition of avocado pear from the control sample (1.18 ml) to sample C (1.54 ml).
|
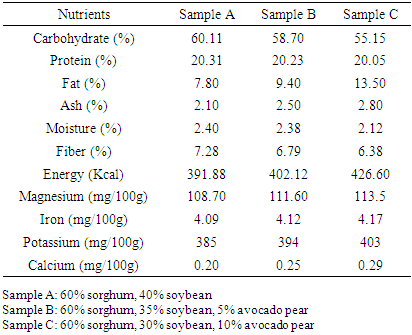
3.2. Nutrient Composition
- The results of the nutrient composition of the complementary diet are shown in Table 2. The protein decreased from 20.31% in the control to 20.05% in the sample containing 10% avocado pear, carbohydrate reduced from 60.11% in the control to 55.15% and fiber content decreased from 7.28% to 6.38% as the quantity of avocado pear added increased. While the energy, ash and fat content increased as the concentration of avocado pear increased. The magnesium, iron, potassium and calcium also increased with the increase in concentration of avocado pear.
|
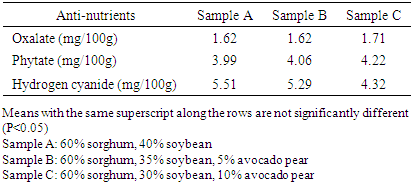
3.3. Anti-nutrients Content
- The cyanide content of the samples reduced from 5.51 in the control sample to 4.32 mg/ 100 g in sample C (Table 3), while oxalate and phytate increased from 1.62 and 3.99 mg/ 100 g in the control to 1.71 and 4.22 mg/ 100g in sample C.
|
3.4. Sensory Qualities
- Preference for colour reduced from 6.3 for the control sample to 5.1 for sample with 10% avocado pear, the most preferred aroma was that of sample A with 6.5 and the least preferred was sample C with 6.1, the texture for the three samples were similarly rated, the preferred taste was that of sample A (control) with 5.2 as mean and the least preferred was sample C (10% avocado pear) with value of 4.8. For acceptability, sample A (control) with mean of 6 was most preferred and the least preferred was sample C (10% avocado pear) with 5.7 as the mean (Fig. 4).
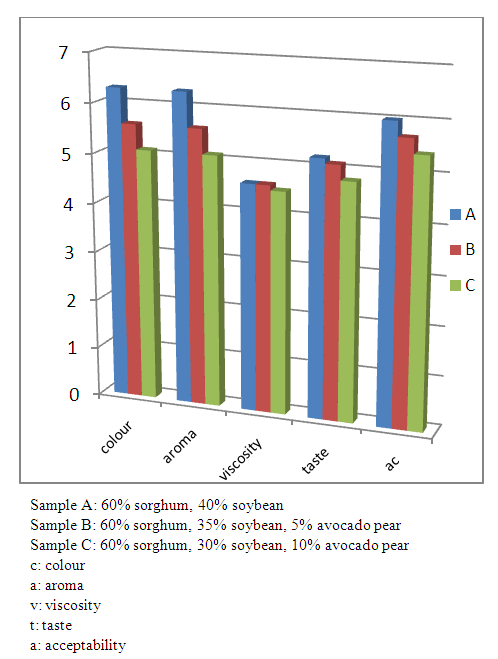 | Figure 4. Sensory qualities of the complementary food samples |
4. Discussion
- There is great importance in producing complementary food from ingredients that are readily available and have suitable nutrients as required for a growing infant especially in developing countries where there is high prevalence of malnutrition. In order to complement the efforts that have been made to produce complementary food that is adequate in nutrients from locally available food commodities, possible inclusion of avocado pear in complementary food was determined. The study formulated complementary food using sorghum, soybean, and avocado pear, where the sorghum and soybean were roasted as previous reported by Adeoye, et al. [23]. There was no significant difference at P<0.05 in the bulk density of the samples which is an indication that the addition of avocado pear had no significant effect on the bulk density. The swelling capacity was recorded as 1.18, 1.32 and 1.54 for the control, sample containing 5% avocado pear and sample containing 10% avocado pear respectively. There was significant difference in water swelling capacity as sample containing 10% avocado pear had the highest swelling capacity which may be highly disadvantageous. Low swelling capacity for complementary food is of prodigious benefit as it increases the nutrient density of the food so the child is able to eat the required amount needed to meet the nutrient requirement [24]. However, the bulk density was comparable to the reports of Ogunka-Nwoka and Mepha [25] and Afam- anene and Ahiarakwem, [24] for formulated complementary food and nutrend, a commercial complementary food and the swelling capacity was lower than what was reported by Adeoye, et al. [23]. Low moisture content of the blend will result in longer shelf life stability. The low moisture content of the complementary food aroused from the roasting method used in the production of the complementary food [26]. The moisture content of the samples reduced with addition of avocado pear with the control having the highest moisture content while the sample containing 10% avocado pear had the lowest. Protein, caloric and micronutrient content of food are important for children. From the study, it was found that the control had the highest amount of protein which is as a result of the higher content of soybean in the sample compared to others. The protein decrease from 20.31 in the control to 20.05% in the sample containing 10% avocado pear, carbohydrate decreased from 60.11 in the control to 55.15% and fiber content decreased from 7.28 - 6.38% as the quantity of avocado pear added increased. While the energy, ash and fat content increased as the concentration of avocado pear increased. The protein content of all the samples was within the requirement for complementary food [27] and fat contribution to the total energy of the complementary food increased from 18% in the control to 29% in the sample with 10% avocado pear. Contribution of fat to total energy was still below the requirement (30 – 45%) for complementary food [27,28]. The micronutrient composition in Table 2 (magnesium, iron, calcium, potassium) increased with the addition of avocado pear with sample with 10% avocado pear having the highest composition. Magnesium and phosphorus were high while calcium was low and iron was comparable to the report of Soronikpoho et al. [29] for complementary food which consisted of fermented bete bete yam, sprouted soybean and millet malt.The anti-nutrient composition (Table 3) of the samples varied, the hydrogen cyanide composition reduced from the control to sample containing 10% avocado pear while the oxalate and phytate content was highest in sample with 10% avocado pear and lowest in the control. The values of oxalate and phytate were lower than what was reported by Adeola et al. [30] but comparable to the report of Soronikpoho et al. [29]. There was no significant change in the sensory qualities with the addition of avocado pear (Fig. 1). The colour, taste, aroma, viscosity and acceptability of the samples had no significant difference. Thus, generally the three samples were acceptable to the panel and inclusion of avocado pear powder did not affect considerably the sensory properties of the complementary food. Avocado pear has proper consistency and texture with a neutral flavour spectrum for first foods. Avocados are promising in helping to meet the dietary needs of infants and toddlers, and should be considered for inclusion in future dietary recommendations for complementary and transitional feeding [31].
5. Conclusions
- Addition of avocado pear to complementary food may be a step further in the quest for formulation of complementary food with adequate nutrients from locally available food commodities.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML