-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2019; 9(1): 7-10
doi:10.5923/j.fph.20190901.02

Antagonistic Effect of Lantana camara against E.coli Versha Upadhyay Uttaranchal PG College of Biomedical Sciences and Hospital, Dehradun
Versha Upadhyay
Uttaranchal PG College of Biomedical Sciences and Hospital, Dehradun
Correspondence to: Versha Upadhyay , Uttaranchal PG College of Biomedical Sciences and Hospital, Dehradun.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

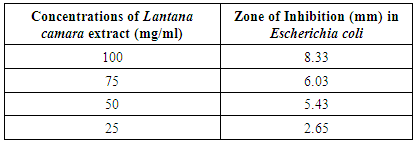
Lantana camara has a wide range of pharmacological activities. E.coli strains and its virulent strains can cause different chronic disease. In this different concentration of extract (100, 75, 50, 25%) gave inhibition zone as 8.33, 6.03, 5.43, 2.65mm. It showed that maximum inhibition at 100% concentration.
Keywords: Lantana camara, E.coli, Microorganism inhibition
Cite this paper: Versha Upadhyay , Antagonistic Effect of Lantana camara against E.coli Versha Upadhyay Uttaranchal PG College of Biomedical Sciences and Hospital, Dehradun, Food and Public Health, Vol. 9 No. 1, 2019, pp. 7-10. doi: 10.5923/j.fph.20190901.02.
1. Introduction
- Medicinal plants have played an essential role in the development of human culture. This is the reasons for the usage of specific medicinal plants for treatment of certain diseases were being discovered; thus, the medicinal plants’ usage gradually abandoned the empiric framework and became founded on explicatory facts. Plants had been the source of treatment and prophylaxis (Hannah Ayrle 2016). Since time immemorial people have tried to find medications to alleviate pain and cure different illnesses. In every period, every successive century from the development of humankind and with the advancement of civilizations, the healing properties of certain medicinal plants were identified, noted, and conveyed to the successive generations Tarek A et. al. (2017). The use of plant compounds to treat infection is an age old practice in a large part of the world, especially in developing countries, where there is dependence on traditional medicine for a variety of diseases (Martins Ekor, 2013). According to World Health Organization, medicinal plants would be the best source of Ayurveda In recent years, antimicrobial properties of medicinal plants are being increasingly reported from different parts of the world (Haidan, 2016). Antimicrobial plant extracts have been recognized as a future source of new antimicrobials in the event of the current downturn in the pace at which these are being derived from micro-organisms. (Desalegn Amenu 2014). Lantana is a perennial, summer-growing, erect or scrambling shrub, growing up to four meters high and often forming dense thickets. Flesh of the plant produces a strong, aromatic odor when crushed. The plant is a member of the Verbenaceae (verbena) family (Sonia, et. al. 2017). Lantana camara has several therapeutic uses, mainly as herbal medicine. (Mauritius, (2018). Plant extracts are used as medicine for the treatment of cancers, chicken pox, measles, asthma, ulcers, swellings, eczema, tumors, high blood pressure, bilious fevers, catarrhal infections, tetanus, rheumatism, malaria and atoxy of abdominal viscera. (Usman et.al., 2007). Lantana camara though being a noxious weed has several minor uses, mainly in herbal medicine. The studies demonstrated that extracts from the leaves can be employed to combat antimicrobial, fungicidal, insecticidal and nematicidal problems. Its potential to serve as biocide has also been illustrated in several studies (Venkatachalam, 2011, Nayak, 2009).
2. Methods
- PLANT MATERIAThe leaves of Lantana camara plant were taxonomically identified from Botanical Survey of India, Dehradun, India. Fresh leaves, mature, immature fruits of Lantana camara were collected from the nearest place of UCBMSH, Dehradun and Fresh leaves, mature and immature fruits of the plants were air dried at room temperature before grinding them to powder form with the help of mechanical grinder. 25 g of the air dried powder of each of leaves, powder were filled in the thimble and extracted successively with 300 ml of methanol using a Soxhlet extractor for 72 h. Each extract was first filtered using Whatman No.1 filter paper. The filtrate was evaporated under reduced pressure in vacuum evaporator. The dried crude extracts were sterilized overnight by UV radiation and stored at room temperature in amber color glass vials until used for antibacterial testing (Boukhris et al., 2013).
 | Figure 1. Methanolic Plant Extract |
 | Figure 2. E. coli strain |
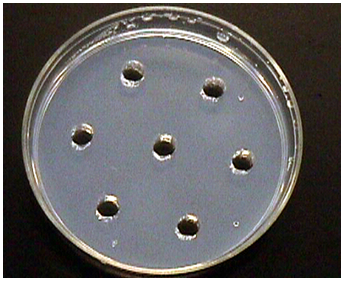 | Figure 3. Plate showing wells made with borer |
 | Figure 4a. Media |
 | Figure 4b. Poured Plate |
 | Figure 4c. Media in test tube |
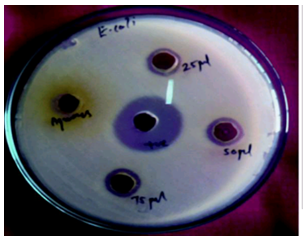 | Figure 4d. Tested poured plate |
3. Result
- Results of the antibacterial screening of different concentrations of the extracts on the test isolates are shown in Table 1 and graph 1. The results show that the increase in concentration of the extract increased the zones of growth inhibition of the bacteria (Fig4d). The assessment of the antibacterial activity was based on the measurement of diameter zone of inhibition (mm) that formed around the hole made by the borer filled with the extract. Maximum inhibition zone was recorded at 100 mg/ml and the minimum inhibition zone at 25 mg/ml in both the bacteria for all the extracts and controlled petri plates showed no zone of Inhibition (Table 1, Fig. 4d and Graph 1).This proved that the extract is effective against microbes.In this study, the results obtained indicated that the methanolic extract of the plants inhibited the growth of the test bacteria. This therefore, showed that the extract contained substances that can inhibit the growth of the selected bacteria. The search for antimicrobials from natural sources has received much attention and efforts have been put in to identify compounds that can act as suitable antimicrobial agents to replace synthetic ones.
|
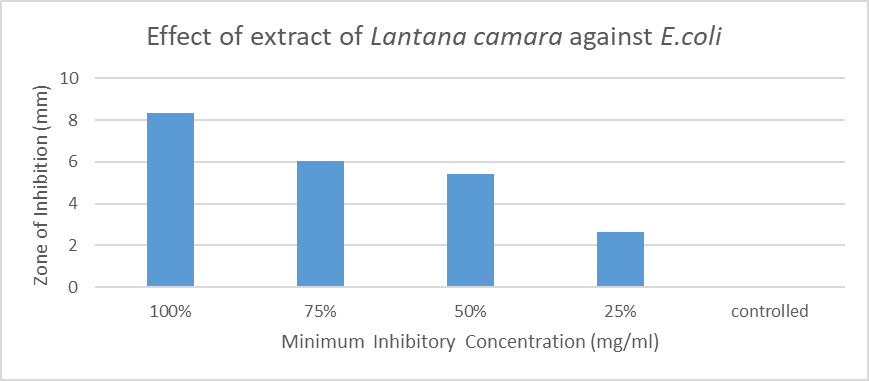 | Graph 1. Effect of extract Lantana camara against E.coli |
4. Discussion
- Phytochemicals derived from plant products serve as a prototype to develop less toxic and more effective medicines in controlling the growth of microorganisms (Yadav et.al. 2016). Medicinal plants constitute an effective source of both traditional and modern medicines (Hosseinzadeh, et.al. 2015). Herbal medicine has been shown to have genuine utility and about 80% rural population depends on its efficacy for their primary health care (Martins Ekor, 2014). Over the years, the WHO advocated that countries should interact with traditional medicine with a view to identifying and exploiting aspects that provide safe and effective remedies for ailments of both microbial and non-microbial origins. Ganesh (2010) observed that extracts from Lantana species able to inhibit the growth of gram-positive bacteria strain.
5. Conclusions
- Above work shown that plant extracts of both traditional and modern are beneficial as antimicrobial, antifungal, antioxidant, insecticidal etc. Maximum phytochemicals which have inhibitory effects on all the microorganism. Phytochemicals are also shown their effectiveness on humans and animals. Plants extract has versatile sources of antimicrobial activity and low toxicity, with low cost and easily accessible to poor communities where the species are found. Costa, et. al. (2007).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML