-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2018; 8(4): 86-94
doi:10.5923/j.fph.20180804.02

Effects of Picking Methods and Cultivation Areas on Biochemical Composition of Leaves from Longleaf Morphotype of Lippia multiflora Grown in Côte d’Ivoire
Fako Kane1, Kouakou M. Dje1, Tia J. Gonnety1, Irié A. Zoro Bi2, Albert Yao-Kouame3, Patrice Lucien Kouame1
1Laboratoire de Biocatalyse et des Bioprocédés, UFR STA, Université Nangui Abrogoua, Abidjan, Côte d’Ivoire
2Laboratoire de Génomique Fonctionnelle et Amélioration des Plantes, UFR SN, Université Nangui Abrogoua, Abidjan, Côte d’Ivoire
3Département des Sciences du sol, UFR STRM, Université Felix Houphouët Boigny, Abidjan, Côte d’Ivoire
Correspondence to: Fako Kane, Laboratoire de Biocatalyse et des Bioprocédés, UFR STA, Université Nangui Abrogoua, Abidjan, Côte d’Ivoire.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The effect of three cultivation areas and two picking methods were evaluated on the biochemical composition of longleaf morphotype of Lippia multiflora (llmLM), to determine how areas and picking method provide leaves with good quality for the production of tea. Results revealed significant variability of leaves composition according to areas and picking method. Leaves from Korhogo were rich in most essential compound such as fat (8.36 mg/100g dw), total phenolic compounds (TPC) (2233.33 mg/100g dw), caffeine (20.97 mg/100g dw), catechin (59.51 mg/100g dw), tannins (14.33 mg/100g dw) and quercetin (1.06 mg/100 g dw), than those from Béoumi and Bondoukou. Fine picking (LsFP) which consist in removing the bud and the two leaves below, provided the highest amount of the majority of compound than leaf samples of coarse picking (LsCP) that concerned picking from buds to fourth leaves below. Ca (7301-10833 ppm), K (1557-7950 ppm), Mg (3060-5449 ppm), Na (1047-1319 ppm) were the most abundant mineral in llmLM leaves. However, result showed that leaves mineral content depend to the cultivation zone. This study revealed that Korhogo is the best area for growing llmLM. Furthermore, fine picking method is the best way to collect the leaves for a good quality tea.
Keywords: Lippia multiflora, Leaves, Biochemical quality, Cultivation area influence, Harvesting method
Cite this paper: Fako Kane, Kouakou M. Dje, Tia J. Gonnety, Irié A. Zoro Bi, Albert Yao-Kouame, Patrice Lucien Kouame, Effects of Picking Methods and Cultivation Areas on Biochemical Composition of Leaves from Longleaf Morphotype of Lippia multiflora Grown in Côte d’Ivoire, Food and Public Health, Vol. 8 No. 4, 2018, pp. 86-94. doi: 10.5923/j.fph.20180804.02.
Article Outline
1. Introduction
- Lippia multiflora belongs to the family Verbenaceae, which is composed of 41 genera with approximately 220 species of herbs, shrubs and small trees [1]. It is one of about 240 plant Lippia species which are extremely plentiful, aromatic and encountered mostly in the tropics of America and Africa [2]. Many species are widely used for their medicinal properties and to season foods. Thus, some of Lippia species are often used traditionally for the treatment of a variety of ailments such as gastrointestinal, respiratory complaints and for relaxation and sedation. Other studies also linked the use of Lippia multiflora to the treatment of blood pressure, malaria, diarrhea and mild hypertension [3]. The leaf decoction is used in Guinea for fumigation and bathing and as a hot application for ear troubles [4]. This plant is commonly known as Lippia tea and commercially known as “Gambian tea ", "Bush tea" and "Healer herb" [4]. Some rural dwellers cook the herbs and use it to relieve stress and enhance sleep [5]. The leaves of Lippia multiflora are used by different tribes as a hot beverage. Seen the importance of Lippia multiflora in the human health, several works were carried out on this plant and were reported in literature concerning the phytochemical screening of the leaves [6] and pharmacological properties [7]. Besides, Obodozie et al. [7] had shown that the leaves of Lippia multiflora possessed analgesic, anti-ulcer, antimicrobial, antiplasmodial and anti-oedema activities, and they are beneficial in the treatment of gastro-intestinal disorders. Otherwise, studies on the biochemical characteristics of Lippia multiflora Moldenke were limited to physicochemical properties of its essential oil [8], the biochemical study concerning its leaves [9] and their chemical constituent evaluation [10]. There is paucity of information on the influence of agricultural and ecological characteristics of the area where the Lippia is cultivated on the elemental composition of leaves. Furthermore, reports of studies from literature show that there exists a great degree of variability in the chemical composition of the oil which may have been due to a number of interacting factors such as geographic origin of plant, environmental factors like stress (soil type, humidity, mechanical damage and cultural practice) as well as genetic factors [1]. Therefore, this study seeks to assess the differences that may exist in leaves from longleaf morphotype of Lippia multiflora (llmLM) as affected by cultivation zone and picking method. We hope this information will be helpful for the valuation of this underutilized plant due to lack of information on its biochemical composition and the appropriate methods of its cultivation and harvest.
2. Material and Methods
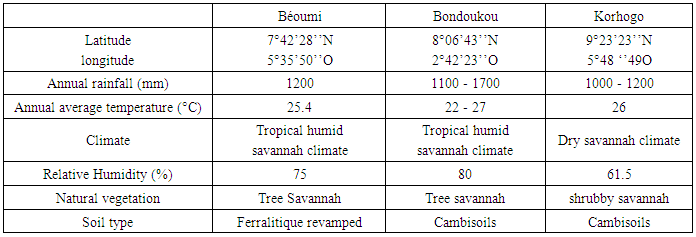
2.1. Description of Study Areas
- The study was conducted on plots set up in three areas of Côte d'Ivoire, including Béoumi (Centre), Bondoukou (east) and Korhogo (north). These localities were chosen on the basis of the differences of their soil and climate characteristic. The characteristics of each of these localities are given in Table 1.
|
2.2. Plant Material
- The fresh leaves of llmLM were collected from domesticated plant in the experimental farms of three regions of Côte d’Ivoire that are "Bondoukou", "Korhogo” and "Béoumi". Then, they were brought to the laboratory for further processing. The harvest was carried out during January to September of 2013 and 2014.
2.3. Methods
2.3.1. Experimental Plots Establishment Method
- The plants were grown in three agro-ecological zones of Côte d’Ivoire. The experimental design was a randomized complete block with three repetitions for each morphotype. Each plot of 400 m2 at each test site was subdivided into 6 subplots each measuring 54 m2 (9 m x 6 m), and containing 45 feet of llmLM. The plots were maintained by regular weeding.
2.3.2. Samples Preparation
- Two picking methods were used to collect the samples of llmLM according methods of He et al. [11]. The first method (fine picking) consisted in appropriating the buds and two leaves that follow and the second method (coarse picking) consisted in appropriating the buds and the four leaves that follow. The different samples were rinsed with clean water and dried in the open air for a week to a constant weight in their respective harvesting locations which environment characteristics are mentioned in table 1. After drying, the leaves were pulverized using a Blender FAR BL514X Cl mixer to obtain a fine dry powder which was stored in sealed plastic boxes and stored at laboratory temperature (28°C) for subsequent analyzes.
2.3.3. Proximate Composition Analysis
- The moisture content in each sample was determined using a hot air oven (Memmert) at a temperature of 105 °C and dried to constant weight according to method AOAC [12]. The ash content was determined by incinerating in a furnace for 5 h at 550°C [12]. Crude protein content was calculated from nitrogen contents (N x 6.25) obtained using the Kjeldahl method by [12]. The fat content was determined by continuous extraction in a Soxhlet apparatus for 8 hours using hexane as solvent [12]. Cellulose content were determined by digesting sample in 1.25% sulfuric acid and 1.25% sodium hydroxide by following the method of [12]. All the analyses were done in triplicate.
2.3.4. Mineral Composition Analysis
- The amount of Ca, Mg, Zn, P, Na, K, Fe, Cu and Mn were quantitatively determined from the digested samples using strong acids and the atomic absorption spectrophotometer with appropriate hollow cathode lamps. Accuracy was assessed by analyzing the samples in triplicate [12].
2.3.5. Secondary Metabolites Analysis
- Total phenolic content analysis: The TPC contents were assessed by the spectrophotometric method described by Singleton et al. [13] using the Folin-Ciocalteu reagent. Phenolics were extracted from pulverized samples of llmLM leaves. Exactly 5 g of samples were weighed out and extracted using 20 mL of 80% (by volume) aqueous ethanol. The mixture was extracted for 20 min in inert atmosphere (N2), filtered through Whatman No 4 filter paper (Whatman International Ltd, Kent, UK) using a Büchner funnel. Extraction of the residue was repeated using the same conditions. The filtrates were combined and adjusted to 50 mL in a volumetric flask with 80% aqueous ethanol. The obtained extract was used for determination of TPC. Extract (0.5 ml) was added with 2.5 ml of reactif Folin-Ciocalteu reagent followed by addition of 2 ml sodium carbonate (Na2CO3) (75 g/l). The sample was then incubated for 5 min at 50°C. The absorbance was then measured at 760 nm using Shimadzu UV-1650 PC Spectrophotometer (Kyoto, Japan). The results were then expressed as mg gallic acid equivalents per gram dry matter (mg /100g) that was derived from a calibration curve determined with different concentration of gallic acid.
2.3.6. Analysis of Phenolic Compounds by HPLC
- Preparation of standard solution: For each sample 1 g of powder was collected in 75 mL of dilute 80% solution and saturated NaCl. After a period of stirring, the solution is put in ultrasonic tank for 30 min to promote the extraction of phenolic compounds. The extracts were centrifuged at 4000 rpm for 10 min using a refrigerated centrifuge (Sigma Aldrich 2-PK type). The supernatant was evaporated at 35°C to 35 mL using a rotary evaporator (Heildolph Laborata 4003 Control, Schwabach, Germany). The remaining solution was then diluted to 100 mL with distilled water. Extraction was performed in triplicate.Qualitative analysis of caffeine and phenolic compounds: The separation of phenolic compounds was performed according to method of Donovan et al. [14]. The sample and standard solutions were filtered through Wattman paper 0.45 µm, and then through millipore membrane 0.45 µm (CARL ROTH, Karlsruhe, Allemagne). The equipment used is a HPLC (Shimadzu, France) system provided with a binary pump (LC-20A) coupled with a UV-VIS detector (SPD-20A). The used column (Thero, Runcom, England) for this analysis was Hypersil ODS type C18, 250 mm x 4.6 mm, 5µm (Thero, Runcom, England). The separation was carried out in elution gradient. The used mobile phase consisted of 50 mM NH4H2PO4 at pH 2.6 (solvent A), acetonitrile solution/ NaH2PO4 (80:20, V/V) (solvent B) and 200 mM o-phosphoric acid at pH 1.5 (Solvent C). The flow rate was 0.5 mL / min. Elution profile was as follows: 100% A for 0-5 min, 92% A/8% B for 5-8 min; 14% B/86% C for 8-20 min; 16.5% B/83.5% C for 20-25min; 21.5% B/78.5% C for 25-35 min, 50% B/50% C for 35-70 min and 100% A for 70-75 min.Each peak detected in the sample solutions was identified by comparing the retention time with chromatogram of reference substance.
2.3.7. Statistical Analysis
- All analyses were carried out in triplicate. Results were expressed by means ± SD. Statistical significance was established using two-way analysis of Variance (ANOVA) models to estimate the effect of picking method and region on proximate composition, mineral constituents and some secondary metabolites of leaves from llmLM at 5% level. Means were separated according to Duncan’s multiple range analysis (P≤0.05), with the help of the software STATISTICA 7 (Statsoft Inc, Tulsa-USA Headquarters) and XLSTAT-Pro 7.5.2 (Addinsoft Sarl, Paris-France).
3. Results
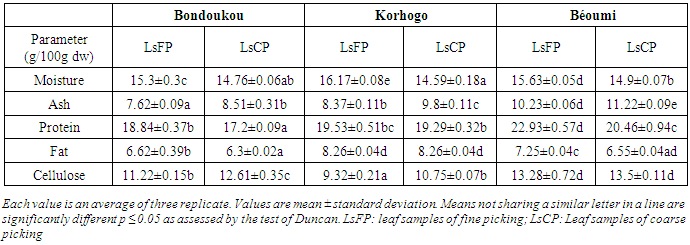
3.1. Proximate Composition
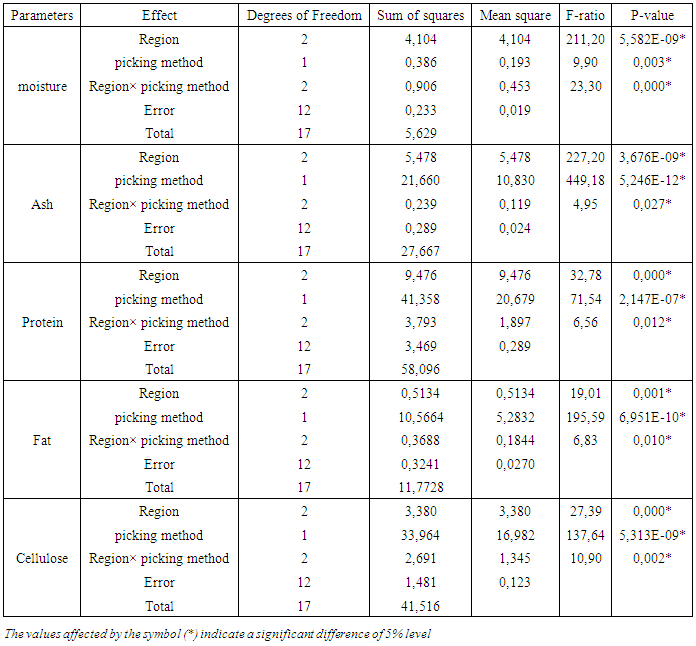
- The proximate composition of leaves from llmLM from three agro-ecological zones in terms of picking method is summarized in Table 2.The moisture, ash, protein, fat and cellulose contents in leaves varied significantly (P≤ 0.05) according to picking method (table 2). Otherwise, the Analysis of variance (ANOVA) showed that picking method and cultivation region main effects and their interaction had meaningful effect (P≤0.05) on moisture, ash, protein, fat and cellulose contents (Table 3). Values of moisture content ranged from 14.59 ± 0.18 g/100g dw to 16.17 ± 0.08 g/100g dw (Table 2). The highest and least moisture content were found in LsFP and LsCP from "Korhogo" respectively. The moisture content in LsFP appeared slightly higher than those in LsCP, whatever is the cultivation area. The ash contents in leaves varied from 7.62±0.09 g/100g dw to 11.22±0.09 g/100g dw (Table 2). The highest ash content was determined in LsCP from "Béoumi", while the lowest was recorded in LsFP from "Bondoukou". Besides, the ash contents in LsCP were slightly higher than those of LsFP. The protein contents in leaves ranged from 17.20±0.09 g/100g dw to 22.93±0.57g/100g dw (Table 2). The highest protein content was recorded in LsFP from "Béoumi", whereas the lowest was observed in LsCP from "Bondoukou". Furthermore, the protein content in LsFP appeared slightly higher than those in LsCP, whatever is the cultivation area.The fat contents in leaves varied from 6.30±0.02 g/100g dw to 8.26±0.04 g/100g dw (Table 2). The highest fat contents were found in LsCP and LsFP from "Korhogo", whereas the lowest was recorded in LsCP from "Bondoukou". Besides, the fat content in LsCP was slightly lower than those obtained for LsFP. The cellulose contents ranged from 9.32±0.21 g/100g dw to 13.50±0.11 g/100g dw (Table 2). The highest cellulose content was determined in LsCP from "Béoumi", while the lowest was obtained in LsFP from "Korhogo". Besides, the cellulose content in LsFP was slightly lower than those of LsCP, whatever is the cultivation area.
|
|
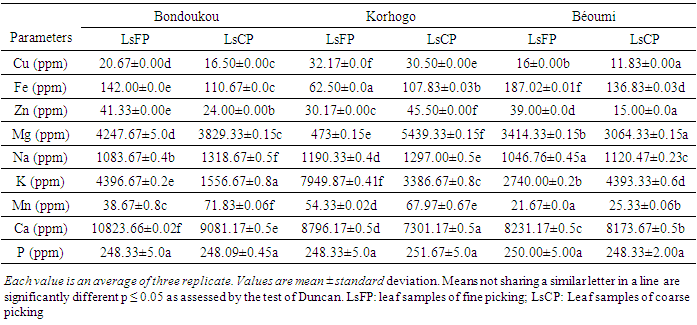
3.2. Mineral Composition
- Table 4 shows some mineral composition in leaves of llmLM from three agro-ecological zones in terms of picking method.The rates of the micro-elements such as Fe, Cu, Zn and Mn varied from 62.50±0.00 to 187.02±0.01 ppm, 11.83±0.00 to 32.17±0.00 ppm, 15.00±0.00 to 45.50±0.00 ppm and 21.67±0.00 to 71.83±0.06 ppm respectively. As for studied macro-elements such as Mg, Na, P, Ca and K, they had respective values in leaves ranging from 3064.33±0.15 to 5439.33±0.15 ppm, 1046.76±0.45 to 1318.67±0.50 ppm, 248.09±0.45 to 251.67±5.00 ppm, 7301.17±0.50 to 10823.66±0.02 ppm and 1556.67±0.80 to 7949.87±0.41 ppm. Besides, the amounts of Cu, Na, Mn and Ca in LsFP were found generally higher than those in LsCP for the same cultivation region. The Analysis of Variance (ANOVA) revealed that picking method and cultivation region main effects and their interaction had significant effect (P≤0.05) on all mineral content except phosphorus content. Significant differences (P≤0.05) were observed between these mineral contents in obtained leaves by the both picking methods.
|
3.3. Total Phenolic Content of Longleaf Morphotype of Lippia multiflora
- The total phenolic contents in leaves ranged from 1190.30±0.26 mg/100 dw to 2233.33±1.28 mg/100 dw (figure 1). The least TPC content (1190.30±0.26 mg/100 dw) was determined in LsFP from "Bondoukou", while the highest value (2233.33±1.28 mg/100 dw) was recorded in from "Korhogo".
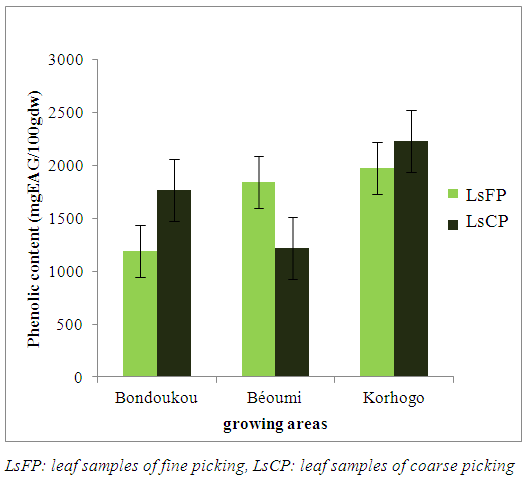 | Figure 1. Total phenolic content of longleaf morphotype of Lippia multiflora according to the picking method and growing areas |
3.4. Some Secondary Metabolites Content
3.4.1. Qualitative Analysis of Caffeine and Phenolic Compounds
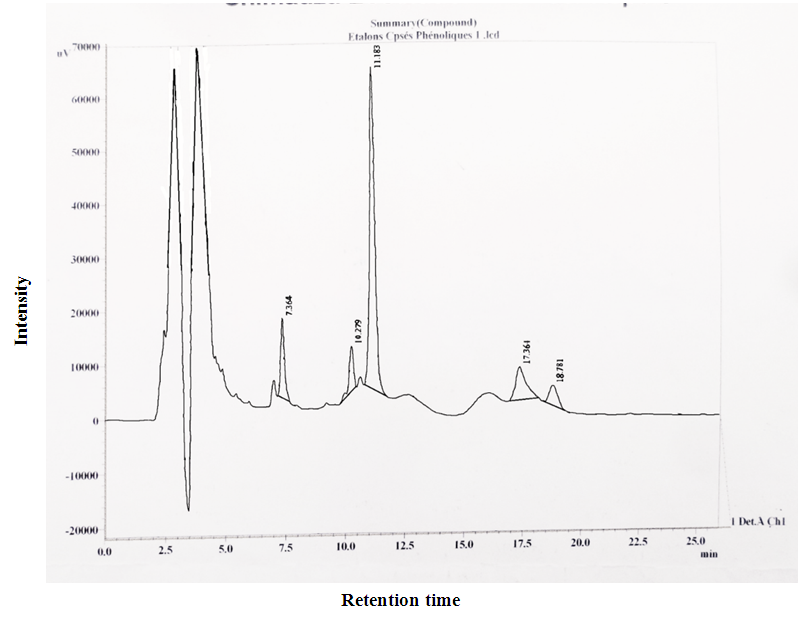
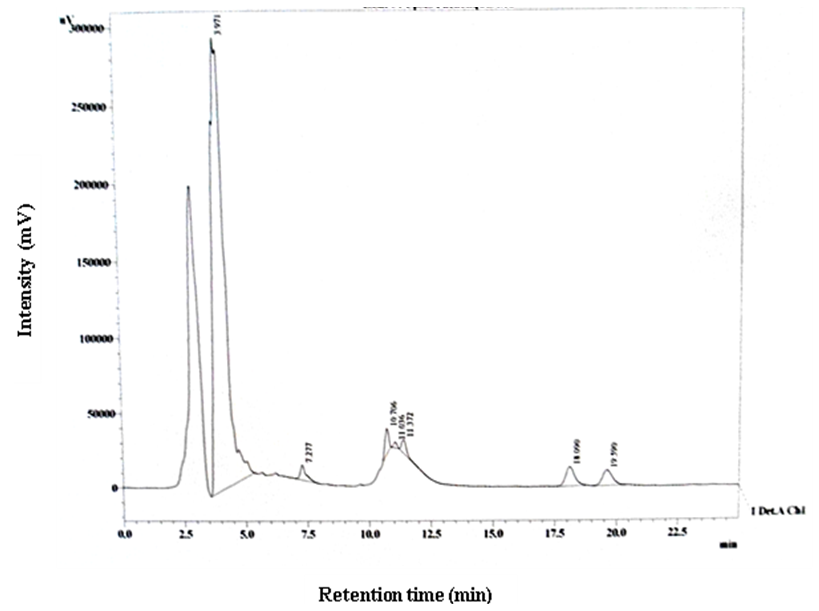
- The representative chromatograms of standard solution and the analysis of the longleaf Lippia multiflora are depicted in figure 2 and figure 3 respectively. Comparing the HPLC chromatogram of the extract of Lippia multiflora with that of the standard solution showed peaks with similar retention times. We note the presence of the flavanone, tannin, quercetin, catechin and caffeine, represented by peaks at 7.36, 10.27, 11.18, 17.36 and 18.78 min, respectively.
3.4.2. Qualitative Analysis of Caffeine and Phenolic Compounds
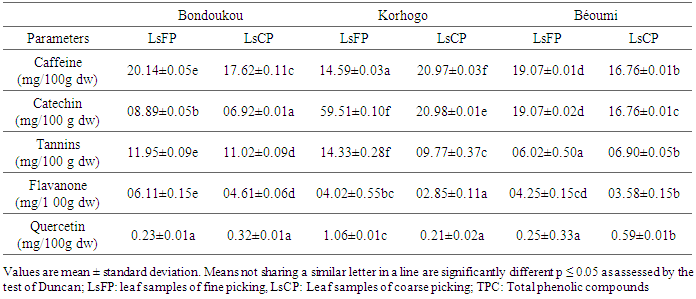
- Caffeine and phenolic compounds contents are depicted in table 5. Results showed significant variability (p<0.05) according to the picking methods and agro-ecological zones.
|
4. Discussion
4.1. Proximate Composition
- The moisture, protein, ash, fat and cellulose contents in leaves from llmLM differed significantly (P≤ 0.05) depending on the picking method, whatever the region investigated.Moisture content of llmLM leaves was higher than the finding of Rahman et al. [15] and Adnan et al. [16] who founded 3.50 to 6.9% and 6 to 7.5% respectively in green tea leaves. This difference in moisture content of leaves could be due to the age of leaves harvested or drying degree of samples [15]. Thus, to ensure proper conservation of leaves of llmLM for a commercial purpose, the water content must be maintained around 6.6% [16]. Variation of ash content could be attributed to the type of soil on which the llmLM is being cultivated, climate, ecological zone and environment [17]. This may be due also to the development stage of leaves. Rahman et al. [15] showed that the ash content of green tea leaves increased with aged leaves. That may explain the high ash level in LsCP than LsFP in all area. Reported ash contents in this study were higher than those obtained with Srilanka tea and Kenya tea by Mohammed and Sulaiman [18], who determined the respective rates of 4.9% and 7.2%. Indeed, the ash content is an indication of its mineral content [7]. Thus, high ash content of LsCP and especially those of Béoumi may express their richness in mineral.The highest amount of protein obtained in LsFP than LsCP corroborates the result of Yao-Kouamé and kané [9] who showed that young leaves of Lippia multiflora, contained more protein than mature leaves. In fact, LsFP contain high proportion of young leaves than LsCP. Protein levels obtained in leaves from llmLM (18.84 – 22.93%), are higher than those reported in previously studies for wild plant Lippia multiflora [19] and Camellia sinensis (Rahman et al. [15]; Adnan et al. [16]). This reflects that the culture of the plant favors increasing the protein content and organoleptic quality tea. Indeed, Ekissi et al. [10] have established a positive correlation between the protein content of leaves and flavor of savannah tea. Moreover, Lee et al. [19] showed that theanine is the most abundant amino acid which contributes to the quality of green tea. Thus, leaf samples from Béoumi, which contain high level of protein, may provide tea with the best flavor.Fat content reported in this study were higher than the findings of Ilyas et al. [20] who recorded fat content of 2.82% in moringa. This may be due to the richness of leaves of llmLM in essential oil [3]. In addition, cultivated condition and soil chemistry affected essential oil level of this plant [21]. This may explain the variation of fat level among different cultivation areas. On the other hand, variability of fat level according to the picking method are in line with the finding of Rahman et al. [15], who showed increasing of lipid with maturation of green tea leaves.As concern cellulose, data showed that its contents in leaf samples of llmLM differed meaningful (P≤ 0.05) depending on picking method and growing localities of plants. This result indicated that the both picking method and locality also influenced the cellulose contents. Our results (9.32-13.50%) were lower than those reported by Adnan et al. [16] for green tea (12.75–17.21%). This lowest of fiber level in this study than those of other teas, may express the best quality of llmLM leaves as tea, because high fiber content is an important quality parameter in commercial tea. In fact, many authors indicated positive correlation between fiber content and quality of tea leaves and proposed a level of fiber less than 16.5% in order to maintain high quality of tea during storage [22]. On the other hand, lowest amount of fiber showed in this study may be due to picking method. Adnan et al. [16] reported that young leaves contained lower levels of crude fiber than mature leaves.
4.2. Mineral Composition
- Result obtained in this study showed ability of llmLM to accumulate considerable level of essential mineral in its leaves. Significant effects of picking method and cultivation localities were observed for all mineral contents except phosphorus content in leaves. Variability of mineral content in L. multiflora leaves according to the origin of the plant was previously reported by Annan et al. [23]. Furthermore, Ca was the most abundant element in leaves from llmLM followed by Mg, K, Na and P, while Fe, Mn, Zn and Cu were present in low level. Similar trends were found in Camellia sinensis leaves by Cao et al. [24]. Our results were in agreement with the result recorded by Adnan et al. [16] who worked on green tea leaf and showed that Ca was the most abundant mineral in the leaf. This richness of llmLM leaves in Ca and various mineral prove their medicinal and nutritional values. Therefore, the leaves could serve as an important source of nutrients.
4.3. Some Secondary Metabolites
- Significant variability (P≤ 0.05) of the TPC, tannins, catechin, flavonoid, quercetin and caffeine contents of llmLM leaves from one picking method to another, whatever the localities, were showed in this study. Data revealed that TPC content of LsFP was higher than those of LsCP for the same region except "Korhogo" region. The variation of TPC contents in leaves among different locations could be explained by the influence of temperature and different prevalent environmental factors [25]. Our results were higher compared to 71.17 µg /mg for Lippia multiflora of Burkina Faso, reported by Dabiré et al. [26]. This high concentration of TPC would express medicinal value of leaves from llmLM, mainly those of Korhogo. Indeed, TPC content in tea leaves are the main indicator for medicinal value due to their antioxidant activities [27]. Several studies reported presence of catechin, tannins, caffeine; flavonoid in leaves of L. multiflora [6, 16]. LsFP from Korhogo displayed the highest percentage in catechin (59.51 mg/100g dw), tannins (14.33 mg/100g dw) and quercetin (1.06 mg/100g dw), than the other area, while those of flavonoid were founded in LsFP from Bondoukou. It is well known that the presence of these compounds in the leaves confers to them certain organoleptic qualities and medicinal properties. Catechin is main component of astringency and a key determinant of tea quality along with caffeine and theanine [20]. Tannins have also astringent properties and in addition, could quicken the healing of wounds and burns [28]. This justifies the usage of L. multiflora in herbal medicine. Wang and Zheng [25] reported that flavonoid was believed to be responsible for antioxidant activity, anticarcinogenic and anti-arteriosclerosis. This compound in tea has high antioxidant and radical scavenging activities. Thus, Korhogo seems to be the most suitable location for growing of llmLM giving leaves with good medicinal properties and best organoleptic quality, while Bondoukou will provide tea leaves with best stimulant properties.
5. Conclusions
- It can be concluded that llmLM leaves are a valuable product based on its rich and beneficial nutrients. They are a good source of protein, TPC and mineral such as Ca, K, Mg, Fe, Zn and Na which are essential for human health and nutrition. High content of phenolic compound (tannin, catechin, quercetin and flavanone) and caffeine are the basis of nutritional, medicinal and pharmacological properties of llmLM leaves. However, the biochemical composition of leaves varied quantitatively according to the cultivation area and picking method. Locality of Korhogo which gave leaves with high levels for most of profitable compounds such as (caffeine, catechin, tannin and quercétine) is the suitable area for the cultivation of llmLM followed by Béoumi. Moreover, the fine picking is the best collection of leaves because it provides high content of most of the components due to its high content of young leaves which are rich in essential organic compounds and mineral.
ACKNOWLEDGMENTS
- The authors would like to thank the International Foundation for Science (IFS) for financial support (grant N° C/5251-1).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML