-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2018; 8(3): 57-64
doi:10.5923/j.fph.20180803.01

Chemical and Nutrition Potential of Amazonian Seeds: Cupuassu and Tucuman
Luiza Silva1, Rutelene Pinheiro1, Ladyslene Paula2, Katia Fernandes2, Antonio Rodrigues1
1Universidade Federal do Pará, Brazil
2Universidade Federal de Goiás, Brazil
Correspondence to: Luiza Silva, Universidade Federal do Pará, Brazil.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The main chemical and nutritional compounds in the kernels of cupuassu (Theobroma grandiflorum) and tucuman (Astrocaryum aculeatum) were evaluated using analyses of centesimal composition, mineral content, ant nutritional factors, amino acid profile, fatty acid profile, and morphology. The chemical composition revealed that both kernels have high lipid content (>43%) made up of saturated and monounsaturated fatty acids, besides all essential amino acids. Potassium, phosphorus, and magnesium were found to be the main minerals present. Among the ant nutritional factors investigated, no hemagglutination activity, cyanogenic compounds, or trypsin or α-amylase inhibitors were found. High phenolic compound contents were found in both kernels. The constituents identified in the kernels are mainly made up of lipids, carbohydrates, and proteins, which makes them foods with high nutritional and energy values. Both can be directly consumed or added to the preparation of food products or may be applied in the food, cosmetic, and pharmaceutical industries.
Keywords: Astrocaryum aculeatum, Theobroma grandiflorum, Amazonian fruits, Centesimal composition, Anti- Nutritional Factors
Cite this paper: Luiza Silva, Rutelene Pinheiro, Ladyslene Paula, Katia Fernandes, Antonio Rodrigues, Chemical and Nutrition Potential of Amazonian Seeds: Cupuassu and Tucuman, Food and Public Health, Vol. 8 No. 3, 2018, pp. 57-64. doi: 10.5923/j.fph.20180803.01.
Article Outline
1. Introduction
- In recent years, an increasing number of studies have been carried out on using seeds and plants as sources of active compounds. Fruits of several palm trees are being studied for their constituents in pulp, skin, and seeds to identify their nutritional quality [1-3].Many seeds have high contents of lipids and proteins, which meet most nutritional requirements of children and adults due to the presence of amino acids and other healthful substances, among which chia, quinoa, and amaranth [4-6]. Edible seeds have been increasingly consumed alone and used to prepare foods, which has produced a promising market. The search for foods with high nutritional value has driven researchers to study materials from unconventional sources for the extraction of compounds of interest [7].Examples include cupulate (a product with nutritional characteristics like those of chocolate) and the fat extracted from cupuassu seeds, which is used as raw material to make cupuassu butter, a replacement of cocoa butter in foods and cosmetics [8]. Another example is the use of tucuman rind and kernel for biochar production and oil extraction, respectively [9]. In this context, cupuassu and tucuman must be further investigated to identify constituents of interest, particularly active compounds with antioxidant properties. To better use these seeds, their anti-nutritional factors, such as amylase inhibitors, tannins, trypsin inhibitors, and phytates, must be determined. These factors are usually found in raw materials of plant origin and may hinder the availability of certain nutrients in the organism, such as the absorption of minerals and proteins, and cause harm to human health [10].Aiming to expand the knowledge on the main chemical compounds and the nutritional value of Amazonian seeds, this study evaluated the fatty acid and amino acid profiles, centesimal composition, mineral content, and ant nutritional factors of cupuassu and tucuman kernels to propose options for their rational use. The study also contributes to minimizing the environmental impact caused by the accumulation of processing waste and driving the socioeconomic development of the region.
2. Material and Methods
2.1. Materials
- The tucuman fruits were collected in two different lots in the city of Colares, 101 km from the state of Para capital, Belém, during the harvest period in March and April of 2016. The fruits attached to the bunches were sent to the Laboratory of Food Analysis of the Federal University of Pará. After selection, the fruits were washed, hygienized and sanitized with 100 ppm sodium hypochlorite solution, followed by depulping and removal of the seeds, which were mixed as a single lot, placed in plastic bags and stored at -18°C.The cupuassu seeds were purchased in 2016 from cooperatives that depulp the fruits in the city of Acara, 162 km from Belem, and sent to the Laboratory of Food Analysis, where they were selected and mixed as a in single lot, washed, hygienized, sanitized with 100 ppm sodium hypochlorite, placed in plastic bags, and stored at -18°C. All chemicals and reagents used in this study were analytical grade and purchased from Sigma-Aldrich Co (St. Louis, Mo., USA).
2.2. Obtaining the Kernels
- After the whole seeds were thawed, they underwent drying at 75°C for 24 h in a forced air circulation oven (Soc Fabbe Ltda, Series 0179, São Paulo, Brazil) and were then broken to obtain the kernels (endosperm). Cupuassu kernels were obtained using a nutcracker and an automated press (Marconi, MA 098/20EL, Piracicaba, Brazil) was used for tucuman. The kernels were ground in a mill (Cadence, MDR 302, Santa Catarina, Brazil) to obtain flour, which was vacuum-packaged (Fastvac, F200, Santo André, Brazil) and stored at room temperature (28 °C) until the analyses.
2.3. Centesimal Composition
- The centesimal composition analyses of cupuassu and tucuman kernel flours were performed in triplicate according to AOAC norms [11] (Method n. 925.09 for Moisture, Method n. 923.03 for Ash, Method n. 945.38 for Fat, Method n. 992.23 for proteins).The results were expressed as mean and standard deviation among the samples. Moisture content was measured in an oven at 105 ± 2°C; lipids, using Soxhlet extraction with petroleum ether; proteins (N x 6.25), by the Kjeldahl method; and ashes, by incineration at 550°C (Fornitec, Model 2017, São Paulo, Brazil). The total energy value (TEV) was calculated based on Atwater conversion factors, described by Osborne and Voogt [12], considering the sum of the energy from the macronutrients and expressed as kilocalories (kcal) [13], where TEV = [4 (Protein + Carbohydrates) + 9 (Fat)].
2.4. Total Phenolic Compounds
- Total phenolic compound contents were determined by the Folin-Ciocalteu method [14] using galic acid for the calibration curve at 0.01 and 0.15 mg.mL-1 (y = 44.32x + 0.0037; r2 = 0.9964) and the results were expressed as mg galic acid equivalent (GAE). 100 g sample-1.
2.5. Mineral Analysis
- The minerals present in the kernel flours were quantified according to methodology by the AOAC (Methods 968.08 e 965.09) [11]. Copper (Cu), selenium (Se), phosphorus (P), magnesium (Mg), manganese (Mn), and zinc (Zn) were analyzed using an atomic absorption spectrophotometer (Shimadzu, AA 6300). Sodium (Na) and potassium (K) were quantified using a flame photometer (Quimis, Q 398 M2, Diadema, Brazil).
2.6. Anti-Nutritional Factors
- Trypsin-inhibitor activity was assessed according to the methodology described by Arnon [15], with absorbance read at 280 nm in a spectrophotometer (Biospectro, BEL SP 2000, Curitiba, Brazil). The activity of α-amylase inhibitors was determined according to Deshpande and Salunkhe [16], with absorbance read at 550 nm and α-amylase activity expressed as units (U), which correspond to the formation of 1 µmol of reducing sugar per minute. Phytic acid content was determined by the method described by Latta and Eskin [17] and DOWEX 1x2-200 ion-exchange resin used according to Ellis and Morris [18]. Absorbance was read at 550 nm and the results were expressed as mg.100 g-1. Tannin content was estimated according to the methodology proposed by the AOAC [19] (Method n. 955.24) using tannic acid as standard, absorbance reading at 760 nm, and results expressed as mg.100 g-1. The presence of biologically important tannins was assessed using the methodology proposed by Cabral, Peixoto Sobrinho, Amorim and Albuquerque [20], with results analyzed for the presence or absence of turbidity.The sample’s hemagglutination capacity was determined according to the methodology described by Moreira and Perrone [21], with results analyzed for the presence or absence of agglutination. The samples were analyzed for cyanidric acid using Guignard’s test, a qualitative technique that confirms the presence or absence of cyanides in toxic extracts. Plum seeds were used as standard to compare the presence of cyanogenic compounds due to their content of cyanogenic glycosides precursors of hydrogen cyanide [22].
2.7. Amino Acid Analysis
- Amino acid profile was determined according to the methodology employed by White, Hart and Fry [23] and Hagen, Frost and Augstin [24]. Quantification was performed by multi-level internal calibration using alpha-aminobutyric acid (AABA) as internal standard. The result was expressed as g.100 g protein-1.
2.8. Fatty Acid Analysis
- Fatty acid conversion into methyl esters (FAMEs) used the method proposed by Rodrigues, Darnet and Silva [25] and detected using a gas chromatograph (Varian, CP-3380) equipped with a flame ionization detector (FID) and a CP-Sil 88 capillary column (length 60 m, internal diameter 0.25 mm, thickness 0.25 mm; Varian Inc., USA). The process conditions were: helium as carrier gas with 0.9 mL.min-1 flow, FID at 250°C, injector (1:100 split ratio) at 245°C, and injection volume of 1 µL. Column temperature was programmed at 80°C for 4 min followed by an increase at 4°C.min-1 until 220°C. The retention time and area of each peak were calculated using the software Varian 3.4.1 and the results were expressed as percentage relative to the total fatty acids.
2.9. Morphological Analysis
- The morphological analyses of the kernel flours were performed in a digital scanning electron microscope (Zeiss, LEO-1430, Hamburg, Germany). The samples were gold coated for 2 min. The analysis conditions for secondary electron images were: electron beam current = 90 µA, constant acceleration voltage = 10 kV, and work distance = 15 mm. The images were acquired and digitized.
3. Results and Discussion
3.1. Centesimal Composition
- Table 1 shows the centesimal composition of cupuassu and tucuman kernels, expressed in dry basis. Both kernels had low moisture content at 1.37 g.100 g-1 for tucuman and 2.13 g.100 g-1 for cupuassu, which indicates the drying process was effective in ensuring microbiological control and extending the product’s shelf life as it reduced water availability for the development of microorganisms and chemical reactions. The ash contents ranged from 1.05 to 3.06 g.100 g-1 for tucuman and cupuassu kernels, respectively. The high lipid percentage found in the kernels (43 to 45 g.100 g-1) makes them possible sources of oil (lipids), and companies already extract oil and butter from tucuman and cupuassu seeds [8].
|
3.2. Phenolic Compounds
- The concentrations of phenolic compounds found in the cupuassu and tucuman kernels were 11,797.0 and 1,178.6 mg GAE.100 g-1, respectively. Those substances have antioxidants properties and, when present in fruits, are also able to kill or inhibit microorganism growth. Jobim et al. [29] reported antibacterial activity against three Gram-positive bacteria and antifungal activity against C. albicans of tucuman pulp extracts and rind. The concentrations of phenolic compounds in the kernels were higher than those of quinoa seeds (71.7 mg GAE.100 g-1) and amaranth seeds (21.2 mg GAE.100 g-1) [5]. The high bioactive compound concentration in the kernels has potential use in foods, cosmetics, and pharmacological formulations, particularly aiming to replace synthetic antioxidants with natural ones.
3.3. Mineral Content
- The cupuassu and tucuman kernels had good content of essential minerals and low sodium content (Table 1). The concentration of potassium, phosphorus, magnesium, and calcium stood out in both samples. Cupuassu kernels had 743.12 mg.100 g-1 potassium, 369.3 mg.100 g-1 phosphorus, 255.13 mg.100 g-1 magnesium, and 103.2 mg.100 g-1 calcium. Tucuman kernels had 202.42 mg.100 g-1 phosphorus, 372.25 mg.100 g-1 potassium, 115.41 mg.100 g-1 magnesium, and 54.20 mg.100 g-1 calcium. The minerals found in the kernels are essential for bone formation and for good muscle function. The concentrations found are higher than those reported for cooked chickpea by Alajaji and El-Adawy [30] of 195, 341, and 165 mg.100 g-1 for P, K, and Mg, respectively. Mineral concentrations of cupuassu kernels were also higher than the ones reported by Jonnala, Dunford, and Chenault [31] for different peanut varieties of 584.20, 199.25, and 83.22 mg.100 g-1 for P, Mg, and Ca. Another noteworthy result in this research is the low sodium concentration found in cupuassu (5.34 mg.100 g-1) and tucuman (7.70 mg.100 g-1) kernels, significantly lower than that reported for peanut (25.88 mg.100 g-1) by Jonnala, Dunford and Chenault [31], which contributes to the use of the kernels as ingredients in food preparations that require lower sodium levels.
3.4. Composition of Anti-Nutrition Factors
- The result of the main anti-nutritional factors investigated in the cupuassu and tucuman kernels are shown in Table 1. No trypsin inhibitors, α-amylase inhibitors, hemagglutination activity, or cyanogenic compounds were found in the samples, thus the kernels may be safely used to prepare foods with no adverse impacts caused by the intake of those anti-nutritional factors that have been widely reported to cause toxicity (cyanogenic glucosides that release hydrogen cyanide) or for interfering with the activity of digestive enzymes, which hampers the digestion and absorption of proteins and carbohydrates [32]. The phytate contents found for cupuassu and tucuman kernels were 127.64 and 97.84 kcal.100 g-1, respectively. The results of this research were satisfactory since the concentrations of phytates were not high, lower than cashew nuts, Brazil nuts, macadamia nuts, almonds, and walnuts, whose concentrations ranged from 150 to 350 mg.100 g-1 [33]. Tannin contents found in cupuassu (153.48 mg.100 g-1) and tucuman (17.04 mg.100 g-1) kernels were low compared with other foods such as raw chickpea (485 mg.100 g-1) and Acacia tortilis (Forssk) seeds (980 mg.10 g-1) [34]. According to Gemede and Ratta [10], some anti-nutritional factors such as phytic acid and tannins may be beneficial to health when used at low concentrations and have been related to reductions in cancer and blood plasma cholesterol levels.
3.5. Amino Acid Composition
- The amino acid profile and chemical score of essential amino acids of the kernels studied are shown in Table 2.
|
3.6. Fatty Acid Composition
- The lipid profiles of the kernels studied are shown in Table 3. The main fatty acids found were stearic, oleic, and lauric.
|
3.7. Morphological Analysis
- The electron scanning micrographs of cupuassu and tucuman kernel flours are shown in Figure 1 and Figure 2. The surface structure of the cells identified in both kernels seem to be coated in a lipid matrix, observed at different magnification levels (250x-2000x), which matches the high lipid content found in the chemical composition of the kernels. Those structures were like the ones observed in papaya seeds in different lipid extraction processes [40].
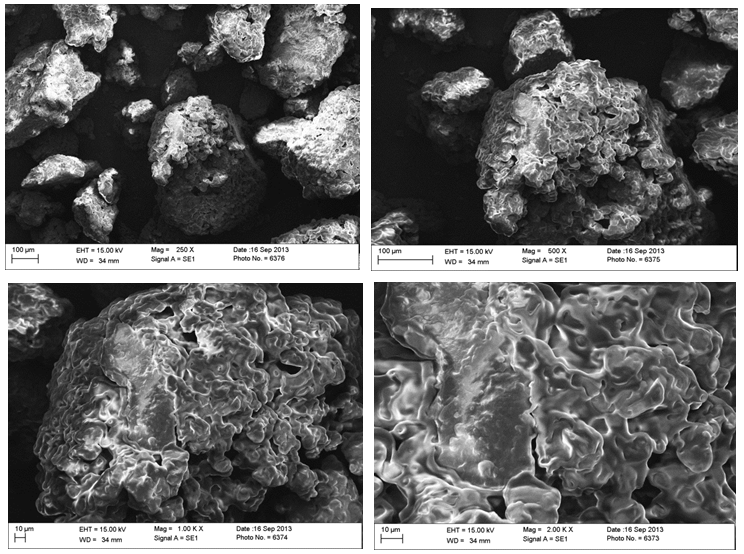 | Figure 1. Micrographs of cupuassu kernel flour at different scales and enlargement |
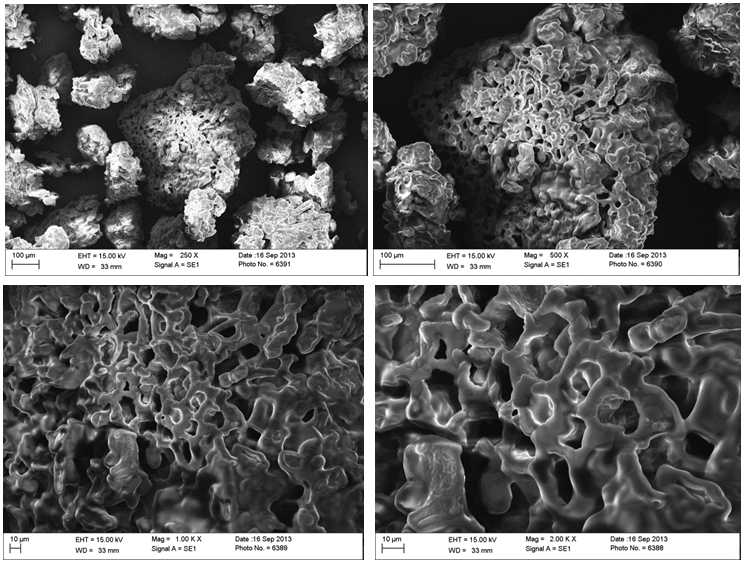 | Figure 2. Micrographs of tucuman kernel flour at different scales and enlargement |
4. Conclusions
- Cupuassu and tucuman kernels are mainly made up of lipids, carbohydrates, and proteins, which makes them foods with high nutritional and energy values. Both can be directly consumed or added to the preparation of food products.Cupuassu kernels proved to be a product with excellent nutritional quality given the presence of oleic and stearic fatty acids and for their antioxidants properties due to the high phenolic compound concentration. They also have all essential amino acids and minerals, particularly potassium, phosphorus, and magnesium. Concentrations of sodium, phytates, and tannins are low, thus they may be used in food preparation.Tucuman kernels showed antimicrobial and antioxidant action provided by the high concentrations of lauric acid and phenolic compounds. The kernel’s nutritional quality is enhanced due to the presence of essential amino acids and three essential minerals (potassium, phosphorus, and magnesium) for muscle and bone formation. The low concentrations of sodium and ant nutritional phytates and tannins allow the kernels to be safely consumed.The kernel constituents are relevant for the food, cosmetic, and pharmaceutical industries, particularly for their antioxidant properties.
ACKNOWLEDGEMENTS
- The authors are thankful to the Graduate Program in Food Science and Technology of the Federal University of Pará, Institute of Biological Sciences II of the Federal University of Goiás, PROPESP/UFPA (Provost’s Office in Research and Graduate Studies), and CNPq (processes 308021/2015-0 477013/2013-9).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML