-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2018; 8(1): 21-33
doi:10.5923/j.fph.20180801.04

Genipap: A New Perspective on Natural Colorants for the Food Industry
Grazielle Náthia-Neves, M. Angela A. Meireles
LASEFI/DEA/FEA (School of Food Engineering), UNICAMP (University of Campinas), Rua Monteiro Lobato, Campinas-SP, Brazil
Correspondence to: M. Angela A. Meireles, LASEFI/DEA/FEA (School of Food Engineering), UNICAMP (University of Campinas), Rua Monteiro Lobato, Campinas-SP, Brazil.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The colors in food attract the attention of consumers, trigger emotions and generate expectations about food. Currently, the use of synthetic colorants is more common than natural ones due to their lower cost and greater stability. However, the long-term use of these synthetic additives can cause serious damage to human health. Currently, no colorant with a natural source is used at an industrial scale for obtaining blue pigments. Therefore, it is highly important to find a new source of blue color because food industries use it in many products, such as ice cream, chocolate and candies, which are mainly products intended for children. This review focused on the use of genipap as an alternative for obtaining a natural blue pigment for use in food industries. Additionally, techniques are described for extraction, and the stability of blue pigments and health properties of genipin are discussed. At the end of the review, it was observed that a stable blue pigment can be obtained from genipap. In addition to coloring, these pigments have medicinal properties of great interest to the pharmaceutical industry.
Keywords: Genipa americana L., Genipin, Color additives, Blue pigments
Cite this paper: Grazielle Náthia-Neves, M. Angela A. Meireles, Genipap: A New Perspective on Natural Colorants for the Food Industry, Food and Public Health, Vol. 8 No. 1, 2018, pp. 21-33. doi: 10.5923/j.fph.20180801.04.
Article Outline
1. Introduction
- Currently, natural products with functional properties have attracted the interest of many industries because synthetic additives are increasingly being replaced with natural additives to attract consumers who have healthy eating habits. Colorants are additives that are present in almost all food products. There is demand from regulatory agencies to reduce the use of synthetic colorants, because they may be responsible for respiratory, epidermal and carcinogenic diseases [1, 2]. In this context, there is an industrial interest in natural colorants, which have limited use in industry due to their instability when exposed to light, pH changes and oxygen [3].Color additives can be classified according to their origin (natural or synthetic), covering (opaque or transparent) and their solubility (dyes or pigments) [4]. Although dyes are soluble in the medium in which they are applied, pigments are insoluble in common solvents [4, 5]. According definition by FDA (Food & Drug Administration) a color additive is any dye, pigment, or other substance that can impart color to a food, drug, or cosmetic or to the human body. Thus, color additives are classified as straight colors that have not been mixed or chemically reacted with any other substance; lakes that are formed by chemically reacting straight colors with precipitants and substrata; and mixtures that are formed by mixing one color additive with one or more other color additives or non-colored diluents, without a chemical reaction. In addition, any chemical that reacts with another substance and causes formation of a color may be a color additive [6].Several vegetable matrices are used to obtain a range of colorants. Annatto (Bixa orellana), for example, is used to extract colors ranging from yellow to red [7]. The extracts from jabuticaba (Myrciaria cauliflora) contain anthocyanins, which are phenolic compounds that generate blue, purple and red colors [8]. The yellow-orange color can be obtained from curcuminoids present in the species Curcuma Longa [9]. However, there is difficulty in obtaining a stable blue color from raw vegetable materials. From this perspective, the genipap (Genipa americana L.), which is a native fruit from Brazil, is an alternative for obtaining a natural blue pigment [10]. The blue pigments from unripe fruits of genipap have been shown to highly stable and have promising applications in food and non-food products [11]. The food industry, for example, utilizes blue coloring in several products and to obtain other colors, such as purple and violet [12].One of the factors that limit the use of natural colorants is their stability. In general, natural additives are less stable than synthetic ones. This instability has encouraged researchers from around the world to search for new technologies applicable to the food and beverage market to obtain non-toxic colorants that are safe to use in food [13, 14]. Thus, this review aims to cover the general aspects of using genipap as a new source for obtaining blue colorants in the food industry. Furthermore, this review includes a brief description of the techniques used for genipin extraction and discusses the challenges faced by the industry in using natural colorants as well as future possibilities for using genipap-based colorants in food products.
2. Colorants in Food
- Color is one of the attributes that is most valued by consumers when purchasing food. To ensure food has an attractive and durable appearance, coloring agents are added to food.Regardless of origin, whether they are natural or synthetic, color additives must: i) Comply with the requirements imposed by regulatory agencies. In Brazil, there are laws that must be followed for the addition of natural and artificial colorants, and a correct description of these additives must be included on the label of food products, such as Decree n° 55871 of March 26th, 1965 [15]; Decree n° 50040, January 24th, 1961 [16]; Resolution n° 37/77 [17]; Resolution n° 44/77 [18]; RDC n° 259/2002 [19]; and Resolution n° 340/2002 [20]. The National Health Surveillance Agency (ANVISA) is the Brazilian organization that regulates the application of 41 food colorants, of which 21 are natural and 20 are synthetic [21], and both types must be within the concentration limits that are necessary for consumer safety [17]. In Europe Union (EU) the regulation (EC) No. 1129/2011 include the rules for food colors; the annexes of the Regulation (EC) No. 1333/2008 contain food categories and a positive list of colors permitted, quantities and instructions for use. Natural pigments should be used in accordance with the rules of the Regulation (EC) No. 178/2002 and other applicable rules [22]. The EU, through Directive 95/45/EC, 1995, authorized the use of 43 colorants in food applications, which includes 17 synthetic and 26 natural colorants [23]. In the United States of America (USA) the rules for food colorants are available under the Title 21 of the Code of Federal Regulations (21 CFR), which contain rules on petitions and labelling and list the specifications and rules for use of approved color additive [22]. The list of colorants permitted in USA is divided into two categories: (i) color additives certified by the FDA, which include 9 additives and (ii) color additives exempt from certification by the FDA, which includes 27 additives for a total of 36 additives permitted by FDA [24].ii) Be stable to prevent degradation of the colorant throughout distribution and sale. The main causes of colorant instability include heat, light, oxygen, acid and exposure to oxidizing agents, such as ascorbic acid and trace metals [3].
2.1. Synthetic Colorants
- Synthetic colorants are produced by complete chemical synthesis or by chemical modification of various precursor compounds [25]. These colorants are widely used in food production because they improve the visual and sensory characteristics of food as well as promoting their marketing. Although they have greater stability, lower production cost and are easier to manage than natural ones, the use of these additives can cause toxic effects at short and long terms for human, e.g. by promoting hyperactivity in children and by their possible carcinogenic effects [11, 27]. Furthermore, the synthetic colorants have been blamed to be harmful to the environment because when they are not being fixed in the food matrix, these colorants pass to the industrial effluent, which when released into water bodies represent a threat to the environment [28].Among the synthetic colorants used in the food industry, azo colorants account for 65%. These colorants are characterized by the presence of nitrogen and provide vivid and intense colors that make their use very common in food, textile, leather and cosmetics [29]. It is estimated that over 10,000 different dyes and pigments are used industrially, and over 7 × 105 tons of synthetic dyes are annually produced worldwide [30].
2.2. Natural Colorants
- The natural colorants obtained from plants, insects, and minerals are characterized by being renewable and sustainable products [31]. The ability to make natural colorants is a technique that has been used since ancient times and has been investigated in recent years due to concerns about the environment and human health [1, 32]. Natural colorants are biodegradable, non-toxic and non-carcinogenic [1]. There have been many advances in developing natural food colorings with respect to extraction processes, purification, stability, identification of new sources, formulation techniques, and hygiene and safety criteria. Nonetheless, there is still a need for developing new natural colorants with high stability and good coloring strength that have wide industrial applications [31]. Currently, several natural colorants are obtained from vegetable matrices. There are many natural colorants applied in commercial foods, these colorants include carotenoids, anthocyanins, chlorophyll and betalains that in addition to providing pigments, perform functional activities in the human body. The chemical structures of some plant-based pigments are shown in Figure 1 [33].
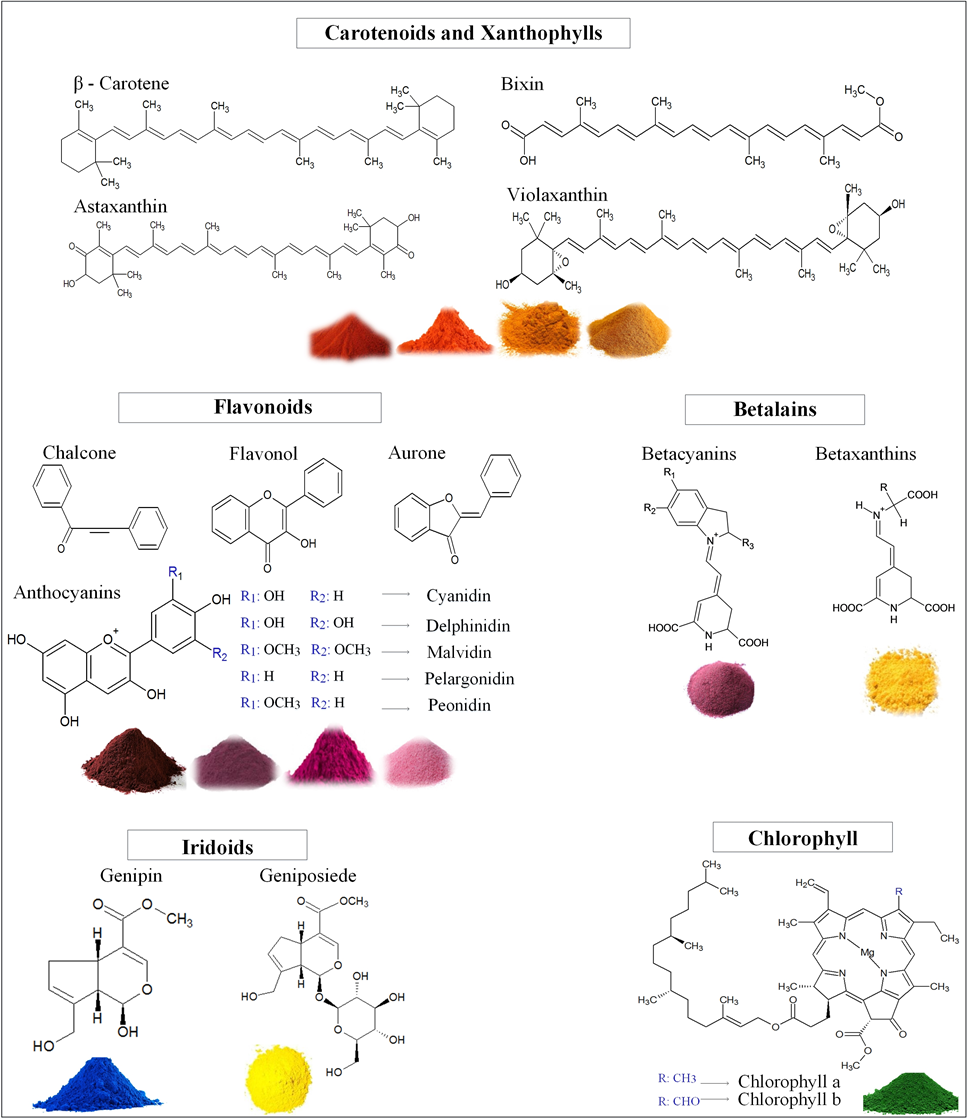 | Figure 1. Natural pigments from different vegetable matrices |
3. Challenges Facing the Food Industry in Obtaining Natural Colorants
- Over the last few decades, a progressive evolution of the food industry has been observed regarding the development of products with natural additives [27]. Motivated by the growing demands of modern consumers, the industrial sector has been increasingly required to offer naturally colored products that not only generate sensory interest but also have potential benefits for human health [26]. The three major challenges industries for obtaining natural pigments are described below [26, 31, 37, 45]:ü Stability: Usually synthetic colorants are more stable than natural ones for a larger range of pH values, temperature variations as well as exposure light and oxygen;ü Efficiency: The efficiency of natural additives is also crucially important for the industrial sector since the amounts of additives must be calculated so that the additive performs its role without decreases in product quality and consumer welfare. Often, higher quantities of natural additives are required compared to synthetic additives and it may not be cost effective or advisable from a health security point of view. Another limitation is the range of tones that are available naturally;ü Cost: The high cost of obtaining natural compounds is another factor that limits the manufacture of products using natural colorants. The cost for recovery and purification of a particular natural compound is often much higher and this cost will be transferred to the final product, which makes it less competitive in the marketplace.Currently, there is no natural colorants production enough to supply the demand of the food industry for natural colorants. Thus, to reach the full production demand needed it is mandatory to invest in research and development in order to find abundant sources of natural colorants which make its application technical and economically feasible. However, it is not enough to develop only a product with appealing color, flavor, appearance, texture and odor attributes. It is necessary that the product provide security for the consumer and not cause harm to their health after ingestion. Therefore, it is necessary for food manufacturers to comply with existing laws. These laws are regularized by different agencies according to each country, e.g., FDA (USA), EFSA (European Union), ANVISA (Brazil). These different laws from each country often represent a barrier to industry since they limit, for example, the marketing of products between different countries.For many years, the use of genipin as a colorant was limited to only a few Asian countries, such as Japan and Korea. More recently, the genipin colorant has been reported as a "fruit juice" color additive in the United States (Title 21 CFR, Code of Federal Regulations, § 73.250) [46] and was approved for food in Colombia [11].
4. Genipap as Source of Blue Pigments
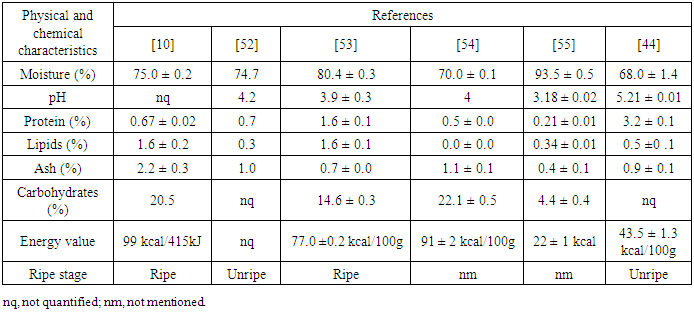
- Genipap is a fruit belonging to Rubiaceae family, which is widely distributed throughout Central America and South America [47]. The fruit has different names according to its place of origin, i.e., in Spanish-speaking regions, it is known as jagua, juito, huito, genipa or caruto, in English-speaking regions, the terms genipap or genipa are names for this fruit, and in Portuguese-speaking regions, such as Brazil, it is popularly known as jenipapo. The genipap tree is an evergreen tree with a height of approximately 10-20 m. The fruits are comestible and globular with a diameter of 5-8 cm and weights ranging from 200-400 g [48, 49]. When in its ripe stage, the pulp is succulent, acidic and hard. The outside of the fruit has a gray-yellowish, dark brown or greenish color [48, 50]. Its pulp is aromatic and mushy.This fruit has numerous albuminous seeds that are hard and have a fibrous consistency with a dark brown color and length ranging from 6 to 12 mm, and the seeds are protected within the fruit by fresh pulp [50]. With an unusual aroma and taste, this fruit is popularly consumed in juices, jams and liqueurs. On the other hand, when in its unripe stage, these fruits may be used as a source of tissue colorants, body paints and food colorings [51]. Figure 2 illustrates the morphological characteristics of the genipap fruit. The physicochemical characteristics of the Brazilian genipap are presented in Table 1.
|
 | Figure 2. Morphology of the unripe genipap fruit |
4.1. Chemical Characteristics of Genipap Fruit
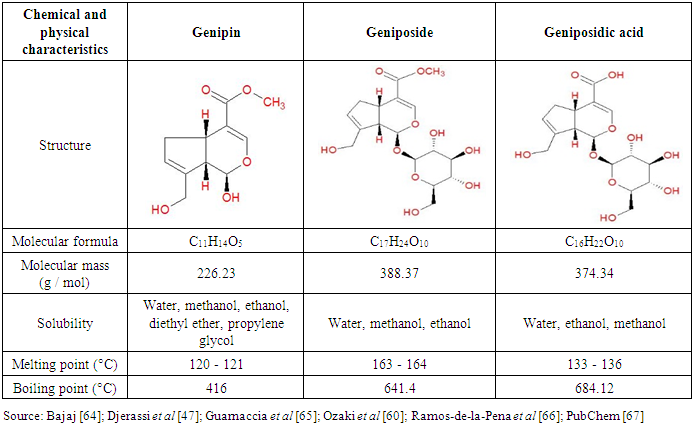
- In terms of chemical composition, genipap is characterized by the presence of three iridoids: genipin, geniposide and geniposidic acid [57]. The iridoids are secondary metabolites usually found in many plants, especially as glycosides. Structurally, they are bicyclic monoterpenes (C10) with a basic skeleton that is a cyclopentane-[C]-pyran ring fused with a six-membered heterocycle oxygenate [58, 59]. Table 2 shows the basic structures of iridoids present in genipap and its main characteristics.
|
4.2. Methods for Genipin Extracting
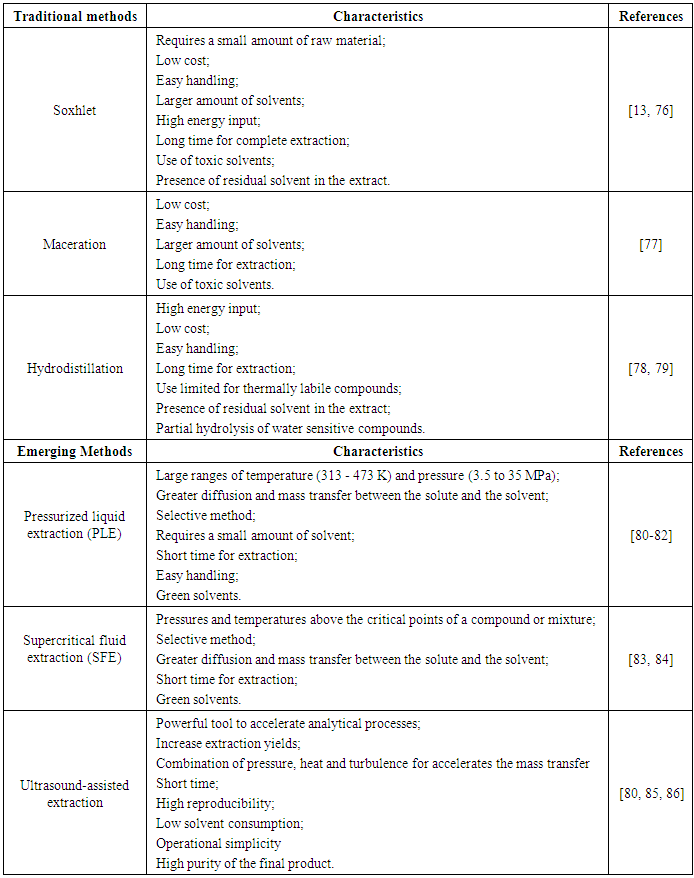
- The biggest challenge of using of genipap as colorant is the techniques for obtaining a high yield of genipin. Several technologies can be used to extract genipin, and selecting the best technology depends on the compound of interest, the available capital and the scale of production. Conventional technologies have already been used for years for obtaining bioactive compounds. However, it is currently necessary to use emerging technologies, which are environmental friendly and promote efficient extraction. Table 3 shows the main features of conventional and emerging technologies.
|
4.2.1. History of the Genipin Extraction
- Attempts to obtain genipin from natural sources have been studied for decades. In 1994, Touyama et al [70] observed that in a hydroalcoholic medium under a nitrogen atmosphere, the reaction between genipin and methylamine (a simple primary amine) produced a yellow pigment and then a red-brown pigment, which changed to blue when reacting to oxygen. This blue pigment consists of a mixture of polymers with high molecular mass that are soluble in water, methanol, and ethanol [70]. In 1996, Penalber et al [87] used different organic solvents to extract blue pigments from genipap. In this study, the extracts obtained with water and ethanol resulted an intense blue colorant that becomes black at temperatures above 80°C. However, the use of hexane as solvent was not able to extract the dye, which may characterize the colorant a polar compound. Five years after, Paik et al [88] studied the stability of blue pigments obtained by mechanical extraction of the dried fruits of Gardenia jasminoides. In this study, the pigments were formed from the reaction of genipin aglycone with the amino acids glycine, lysine, and phenylalanine. The experiment was performed under different pH (5.0, 7.0 and 9.0), temperature (60, 70, 80 and 90°C) and light intensity conditions (5000, 10000 and 20000 lux). Among the amino acids used lysine generated the largest remaining percentage of blue pigments after 10 h at 60°C. At pH 5.0, 7.0 and 9.0, the percentage of remaining pigments was 104, 102 and 110%, respectively. This outcome indicates that the amino group of lysine plays a crucial role in the formation of blue pigments. Unripe fruits of genipap were subjected to mechanical extraction in the presence of water and aqueous ethanol at 50% and 95% by Renhe et al [12]. These authors assessed extraction at different pH values (4, 5, 6, 7, 8 and 9) and different temperatures (35, 45, 55, 65 and 75°C) at a ratio of 1:2 (a part of the fruit to two parts solvent). The extracts that were obtained were analyzed by colorimetry, and it was concluded that the temperature contributed to the formation of blue color. By increasing the temperature, the extract acquired a black color. The optimal conditions of the extraction with water were at 55°C and pH 4.0. The ethanol solutions had better performances at a temperature of 75°C and pH 4.0. Processes for obtaining and applying blue colorants from genipap have already been patented by some authors. Wu et al [89] patented a method (US20090246343 A1, publication date in Oct. 1st, 2009) for producing natural stable color products by adding some edible materials to the juice of genipap. In this study, the authors used the ripe fruits of genipap and different shades of blue, green and purple were observed as well as the brown and black colors. The products generated in this experiment showed excellent stability under acidity and heat, which allowed the products to be used in food, beverages, medicines, dietary supplements, cosmetics, personal hygiene materials and animal feed. A process for obtaining blue color was also patented by Echeverry et al [90] (US7927637 B2, publication date in Apr. 19th, 2011). In this study, the pulp was separated from the fruit and subsequently milled. Afterwards, the raw liquid juice was mixed with glycine. This mixture (juice and glycine) was heated for 2 hours at approximately 70°C. Then the extract was dehydrated using a lyophilization to produce a solid blue colorant. Color compounds were isolated from the reaction of genipin from the genipap fruit with glycine. This study was patented with the number US20130345427 A1 and published in Dec. 2013. The aim of this research was to study the molecular structure of the blue pigment resulting from the reaction. The unripe fruit of genipap was freeze-dried and extracted by Soxhlet with dichloromethane. After extraction, the solvent was removed and genipin was identified by thin layer chromatography. Then glycine was dissolved in an aqueous medium at 70°C. A solution of genipin and methanol was added to this mixture and stirred for 4 hours. After the reaction, the mixture was lyophilized, and the blue powder was extracted with ethyl acetate to remove excess genipin and other polar compounds. Finally, the fractionation was performed by chromatography analysis of the materials resulting from the reaction [91]. Wu and Horn [92] patented a method (US8945640 B2, publication date in Feb. 3th, 2015) of producing extracts rich in genipin from genipap. The extraction developed by these authors involved the use of aqueous solvents (polar) and organic solvents (non-polar). First, the fruits were washed and then peeled. Water was used as a solvent and the mash that was obtained from the mixture (solvent + fruit) was filtered on a filter press to separate the solids. The pH was adjusted to 3.8-4.0, and the extract was concentrated in vacuum rotaevaporator. A second extraction with non-polar solvent was performed. The organic solvent was separated from the aqueous phase by decantation, and the organic phase was separated using a high speed centrifuged. The solvent was removed by evaporation and a solid extract rich in genipin was obtained (70% w / w). To obtain the colorant, the authors evaluated the use of the amino acids L-threonine, L-isoleucine, and L-histidine in the ex-tracts and observed that after heating, the amino acids L-threonine and L-isoleucine generated a green color, while the blue color was formed when L-histidine was added. The addition of L-alanine and xylose provided an extract with a red-orange color for the extract. Most of the studies are limited to color analysis. Only in recent years have some studies examined the extraction conditions of the process and extraction yield.Genipin was obtained from genipap by solid-liquid extraction by maceration of unripe fruits using chloroform at ratio of 1:2 (a part of the fruit to two parts of solvent). The yield of genipin obtained from unripe fruits stored on refrigeration (T < 0°C) for 41 days was 0.44 ± 0.06%, a yield 15 times higher than that obtained using freshly collected unripe fruits. These authors in their experiment observed color changes, where the extracts from the fresh fruits were greenish-white color while the extracts from fruits stored for 41 days were blue [69].Obtaining genipin with ultrasound treatment was studied by Ramos-de-la-Pena et al [66]. In this study, the samples of genipap were submitted to temperatures of 5, 10 and 15ºC for 5, 10 and 15 minutes (285W, 24 kHz). The results obtained after cold-extraction showed that the process performed at 10°C for 15 min was the most efficient in terms of the yield of non-crosslinked genipin (7.9 ± 0.3 mg / g of the fruit). Ramos-de-la-Peña et al [74] studied the recovery of genipin from genipap by high pressure processes combined with enzymatic treatments. Among the tested conditions, the pressure of 130 MPa provided the highest yield at the temperature of 9.3 ± 0.5°C without the addition of pectic enzymes. The yield obtained at these conditions was 34 ± 2 mg / g of fruit.The genipin extraction with pressurized ethanol was studied by Náthia-Neves et al [93] in several parts of the unripe genipap fruit. In this study, the authors observed that the endocarp presented with the highest recovery of genipin (48.6 ± 0.6 mg/g raw material) at 80°C and 12 bar.
5. Benefits to Human Health
- As stated before, the geniposide releases aglycone genipin after hydrolytic cleavage by a β-deglycosidase enzyme in the human intestine. This iridoid has some pharmacological effects, such as activity against oxidative damage and inhibition of tumors [94]. In addition, several authors have called attention to the biological properties of genipin since this compound is able to act as an antimicrobial and anti-inflammatory agent [95, 96] in addition to having antilipoperoxidative [95] anti-cancer [97] anti-diabetic [98, 99], and antioxidant activity [100] as well as protecting against liver (hepatic) diseases [101] and protecting hippocampal neurons [102, 103]. These compounds also have antithrombotic [104] and neuroprotective effects [105, 106].
6. Conclusions
- At the end of this review, it could be concluded that it is possible to obtain stable natural blue colorant using unripe fruits of genipap as a raw material. Although there are some limitations, the use of natural colorants has been increasingly encouraged due to health benefits and the quality of the final product. There are few studies regarding the yield of genipin from genipap fruit extraction. Additionally, there are no reports regarding the economic assessment of implementing this compound at an industrial scale. The lack of studies involving the extraction and application of genipin as a colorant agent can be related to the fact that using this agent in food is permitted only in some countries.However, further investigations should be carried out before these colorants are used in industrial applications, among which should be:ü Develop more in vitro studies to ensure that the colorants obtained from genipap present no risk to human health;ü Use the colorants in different products and evaluate their stability and sensory quality at all stages of the production chain (i.e., production, transportation and marketing);ü Develop processes that allow blue pigments to be obtained with high purity and yield for the product to be competitive in the global market and ensure the processes are environmental friendly.
ACKNOWLEDGEMENTS
- G. Náthia-Neves thanks CAPES/DEA/PROEX for Ph.D. assistantship and M. A. A. Meireles thanks CNPq for the productivity grant (302423/2015-0).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML