-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2017; 7(4): 98-105
doi:10.5923/j.fph.20170704.04

Effect of Duck Egg -Infant Milk on Body Weight in Babies with Undernutrition. A Randomized, Double Blind, Controlled Trial
Ram B. Singh1, Rajneesh Kumar2, Rajesh Singh2, Toru Takahashi3, Germaine Cornelissen4, Fabien De Meester5, Agnieszka Wilczynska6, Jan Fedacko7, Krasimira Hristova8, Galal Elkilany9, Sergey Chibisov9, Telessy Istvan10
1Halberg Hospital and Research Institute (HHRI), Moradabad, India
2Consultant Pediatrics, HHRI
3Graduate School of Human Environmental Science, Fukuoka, Japan
4Halberg Chronobiology Center, University of Minn, Medical School, Minneapolis, USA
5The Tsim Tsoum Institute, Marche, Femenne, Belgium
6The TsimTsoum Institute, Krakow, Poland
7PJ Safaric University, Kosice, Slovakia
8National Heart Hospital, Sofia, Bulgaria, Gulf Medical College, Ajnam, UAE
9People’s Friendship University of Russia, Moscow, Russia
10Pécs University, School of Pharmacy, Dept. Pharmaceutics, Hungary
Correspondence to: Fabien De Meester, The Tsim Tsoum Institute, Marche, Femenne, Belgium.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background. Epidemiological studies indicate that in 2015, 4.5 million (75% of all under-five deaths) occurred within the first year of life. Cohort studies revealed that in lower middle income countries, one third of the babies below 6 years are under weight, and in urban areas there is increase in childhood obesity due to increased intake of high glycemic index infant milk and other ready prepared foods. It is possible that a wild type egg, with lower glycemic index and high nutrient density may be a better choice to those infants who are not able to get breast milk and/or have under-nutrition. This study aims to examine the effect of a newly developed milk formulation based on wild duck egg compared to dairy milk on body weight, waist circumference and blood glucose in children with under-nutrition. Study Design. Randomized, double blind, controlled trial. Setting. Primary and Secondary care Hospital. Subjects and Methods. After approval by the ethics committee and obtaining parental informed consent, all the babies (below two years age; n=20) with under-nutrition (defined by percentile of body weight) were randomized to take ovolipids’ infant milk (800 ml or 100 g) or dairy milk (800 ml) /day, for 10 and 28 days. Body weight was measured by a digital scale in under-clothes, and waist circumference and height were determined in the lying position, before and after the 10- and 28-day trial. Blood glucose was measured by glucometer by a finger prick at the end of the 10–day trial. Babies were allowed to take whatever they are consuming normally. Food intakes and physical activity were assessed by validated questionnaire, filled with the help of mothers. The infant milk formulation (Ovolipids IF) was based on guidelines of the ESPGHAN (WHO 2005). It contained 2.4gP/100Cal (10%), 5.2gF/100Cal (45%), 11.5gC/ 100Cal (45%), based on duck egg, whey proteins and glucose syrups. Results. The DIEM trial shows thatat 4 weeks, weight gain was statistically significant in both groups, but was higher in the ovolipid than in the dairy group (+0.45 vs. 0.26 kg, P = 0.027). Corresponding changes in waist circumference were also significant: while it decreased in the ovolipid group, it increased in the control group (-2.0 vs. +1.7 cm, P < 0.001). Post-prandial mean blood glucose was significantly lower in the intervention group than in the dairy milk group (85.9 ±2.6 vs. 98.5 ± 4.4 mg/100ml, P<0.001). The tolerance of ovolipid milk was excellent, both during morning and evening administration (about 500-600 ml at a time in 3-4 hours). Side effects were loose motions in two cases, in each group, during the 28 days of follow up. Conclusions. Ovolipid consumption asdaily infant milk may cause possible greater increase in body weight with a possible decrease in waist circumference and significant decline in postprandial blood glucose which are indicators of metabolic syndrome.
Keywords: Formula food, Metabolic syndrome, Rapidly absorbed foods, Diet, Obesity
Cite this paper: Ram B. Singh, Rajneesh Kumar, Rajesh Singh, Toru Takahashi, Germaine Cornelissen, Fabien De Meester, Agnieszka Wilczynska, Jan Fedacko, Krasimira Hristova, Galal Elkilany, Sergey Chibisov, Telessy Istvan, Effect of Duck Egg -Infant Milk on Body Weight in Babies with Undernutrition. A Randomized, Double Blind, Controlled Trial, Food and Public Health, Vol. 7 No. 4, 2017, pp. 98-105. doi: 10.5923/j.fph.20170704.04.
1. Introduction
- Epidemiological studies indicate that undernutrition as well as over-nutrition have adverse effects on future risk of obesity and metabolic syndrome [1-4]. WHO and UNICEF expert reports based on cohort studies indicate that globally, the infant mortality rate has decreased from an estimated rate of 63 deaths per 1000 live births in 1990 to 32 deaths per 1000 live births in 2015 [5, 6]. Annual infant deaths have declined from 8.9 million in 1990 to 4.5 million in 2015 and in 2015, 75% of all under-five deaths (n= 4.5million) occurred within the first year of life [5, 6]. Apart from these effects, among the 75 countries with the highest burden of child deaths, 40% of such deaths occur during the neonatal period [5, 6]. In India 28% children below 6 years are under weight, and in UP state, the fraction of underweight children is 35.5% [6]. These studies indicate that infant mortality and morbidity due to malnutrition has become a public health problem, despite tremendous efforts made for prevention. There is a need to find new solutions to improve public health, by participation of public for prevention of undernutrition.A cohort study from Norway, showed that childhood poverty followed by a high standard of living operates, at least partly, as a risk factor for coronary artery disease (CAD) through conventional risk factors [1]. The Bogalusa Heart study revealed that childhood obesity is related to adult levels of obesity, lipids, lipoproteins, blood pressure, and insulin and to morbidity from CAD [2]. However, the importance of the age at which obesity develops in these associations remains uncertain. Further studies have shown that impaired growth in infancy and rapid childhood weight gain exacerbate the effects of impaired prenatal growth resulting in obesity, metabolic syndrome, cardiovascular disease (CVDs) and type 2 diabetes in adult life [3]. A later study reported that low birth weight, a risk factor for CVDs in later life, is already associated with elevated fetal glycosylated hemoglobin at birth [4].Infant milk consumption by infants has been associated with insulin resistance and metabolic syndrome later in adult life. In United States and other developed countries, health outcomes differ substantially for mothers and infants who formula feed, compared with those who breastfeed [7]. Those infants, who have not been breastfed suffer an increased incidence of infectious morbidity, including otitis media, gastroenteritis, and pneumonia, as well as elevated risks of childhood obesity, type 1 and type 2 diabetes, leukemia, and sudden infant death syndrome (SIDS) [7]. The cause may be a high glycemic index, with unbalanced nutrient content of the milk, available in the market, for the last 4-6 decades. Thus the nutrition among infants and children may be responsible for triple burden of diseases; during infancy, childhood and later adult life. Therefore, a new opinion of optimal early human development is emerging which takes account of both short and long-term outcomes in relation to future health and development of non-communicable diseases (NCDs). The effects of high glycemic index Western foods, on risk factors for CVDs and type 2 diabetes need further studies in the light of high glycemic index of foods. We understand that due to refining and amount of sugar in the foods, some carbohydrate-rich foods have less effect than others to increase blood glucose which is called glycemic index which is associated with greater risk of obesity, central obesity and type 2 diabetes as well as vascular disease [8]. In the present study, we examine the effect of a newly developed milk formulation based on wild type duck egg [9], in children with under-nutrition to find out its effects on body weight and overall development.
2. Subjects and Methods
- The DIEM trial was conducted at Halberg Hospital and Research Institute, Moradabad, India and the study was approved by the ethic committee of this hospital. After obtaining parental informed consent from each subject, all the children below two years of age were invited for screening and recruitment to the study by advertisement in the clinics and nearby streets.Recruitment of ParticipantsDuring the recruitment period of 7 days, 32 babies from two streets, came for screening, to participate in the trial. Of 32 babies, 4 were not suitable for screening due to age above 2 years and 3 parents refused to come daily for feeding.Exclusion criteriaOf remaining 25 children below two years of age, 3 were excluded due to past history of frequent loose motions (n=2) and vomiting (n=1) and 2 developed loose motions or vomiting during the run in period of 7 days. Inclusion criteriaEligible participants (n=20) below 2 years of age, with the diagnosis of under-nutrition based on WHO criteria; children with a weight-for-height above -1 SD (known to have a lower risk of death and are advised to be managed at home by the WHO. (http://www.who.int/nutrition/publications/severemalnutrition/9789241598163/en/). All the participants completed a 7-day run-in phase during which each study milk was given in the morning or evening. During run-in periods, participants were provided all of their meals, snacks, and calorie-containing beverages. RandomizationRemaining 20 participants with under-nutrition, were randomized to intervention (n=10) and control group (n=10) by computer generated random numbers, created by a computer engineer, unconnected with the study. The intervention group received ovolipids’ infant milk (800 ml or 100 g) and the control group, dairy milk (800 ml), for 10 and 28 days. The remaining milk was consumed during other parts of the day. All the participants were allowed to continue taking other foods which they were consuming earlier. The experimental intervention group and control group, as well as experts measuring the demographic data and blood glucose were blind to treatment to maintain blindness of the trial.Study Agents (Milk)Experimental Intervention Group: The infant milk formulation (Ovolipids IF) was based on guidelines of the ESPGHAN (WHO 2005) [10]. It contained 2.4g proteins/100Cal (10%), 5.2g fat/100Cal (45%), 11.5g carbohydrates/ 100Cal (45%), based on wild type duck egg, whey proteins and glucose syrups (Total =625 Cal/day) which are rich sources of omega-3 fatty acids as well as essential and nonessential amino acids [9].Control Group; received dairy milk containing (each 100g), energy 57.8 Kcal, total fat 3.0g, saturated fat 2.0 g, total proteins 3.0 g, total carbohydrates 4.7g, without any added sugar. (Total 800ml/day=462.4 Kcal/day)Both the groups were admitted in the hospital free of cost, for 3-4 hours in the morning and in the evening to ensure maximum feeding of the intervention and control milk and the groups were assured free treatment if there is any side effects. Both groups were paid transport charges to facilitate the attendance in the hospital.Follow upThe feeding of both types of milk was reinforced daily by the dietitian to maintain the compliance, during the follow-up of 10 days and 28 days. The hospital staff visited the house of the babies, to motivate the parents to come to the hospital for feeding, if they had any inconvenience in coming. In a few subjects, the hospital staff visited the house of the babies for feeding and collection of data, when they did not come on persistent request, during the follow up of 4 weeks.MeasurementsPersonal interviews of the parents were conducted to collect the information by validated questionnaire filled with the help of mothers to find out the food intakes, physical activity and time of eating other foods.Dietary intakes were obtained by filling dietary diaries by the parents and by the dietitian for 3 days, by asking probing questions and by using food measures, food models and food portions. Total energy intakes per day were calculated by finding out energy and nutrient content of foods from the Indian food composition tables [11]. Physical activity was assessed by finding out the duration of sleep in the night and day time and duration of playing in hours by validated questionnaires, based on diaries completed by parents and our trained staff considering the previous day. The questionnaires were on time of walking, sitting, playing and sleeping during the last 24 hours, which were recorded, while working individually with children.Body weight, length and waist circumference were measured during recruitment before entry to the study and during follow up at 10 days, weekly and at 28 days of trial. Body weight was measured by a digital scale in under-clothes and length was determined in the lying position, by a tape. The waist circumference was also measured in the lying position in the morning after defecation to avoid gaseous distention from the measurement. Blood glucose was measured by glucometer by a finger prick at the end of the 10–day trial.Statistical AnalysisChanges in weight, BMI, waist circumference, and blood glucose concentrations after 4 weeks were compared between the Ovolipid milk intervention group and the dairy milk control group with the t test. In view of the direction of changes was anticipated, 1-tail tests were used. Two-way ANOVA were used to assess the effect of both milk formulation and time of feeding and their interaction. Paired t tests carried out on each milk formulation and feeding time subgroup assessed whether weight, BMI, waist circumference, and blood glucose concentrations changed with statistical significance. Results were deemed statistically significant if the P-values were less than 0.05. The results from the 2-way ANOVA: for BMI, both factors were statistically significant at 5%. Since the direction of the difference was anticipated, Student t tests can be evaluated with 1-tail instead of 2, in which case results are also significant at 5%.
3. Results
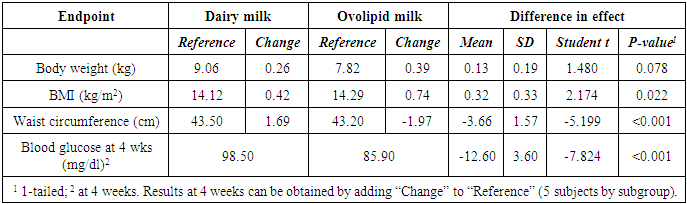
- Of 32 respondents, 20 volunteers (n=10 Ovolipids vs 10 dairy milk group) below 2 years age were included in this study and rest were excluded as described earlier. There were 6 males in the control group and 2 in the experimental intervention group and rest subjects were females (Table 1). Mean age and mean body weight at entry to the study, were significantly higher in the control group receiving dairy milk compared to ovolipids intervention group but the BMI showed no significant difference between the two groups.
|
|
4. Discussion
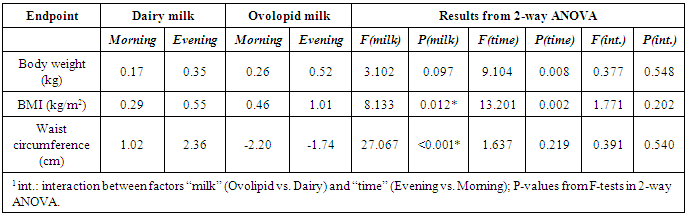
- Food security for mothers have reduced the problem of undernutrition and low birth weight among newborn and infants, but it continues to be a major public health problem in more than 75 countries of the world [5, 6]. Reduced availability of breast milk due to poor health of mothers may be responsible for undernutrition and infection among newborn and infants, whereas increased intake of high glycemic index ready prepared infant foods, may be the cause of obesity among children [7, 8]. The glycemic index of foods is quantified according to the amount 50 g of its carbohydrate compared with 50 g of glucose which increases blood glucose during 2 hours [8]. The effects of glycemic index, on blood glucose may also result from other nutrients, such as fiber, proteins, fatty acids, potassium, magnesium, zinc, chromium and polyphenols, which favorably affect health [8, 9]. There is a need to develop new formulation infant foods, similar to a wild type egg, which may have a lower glycemic index and include necessary nutrients; high omega-3 fatty acids and flavonoids as well as essential and nonessential amino-acids which are important for the development of immunity, brain and overall development of the infants [9]. It may be substitute to those infants and children who are not able to get breast milk and/or have under-nutrition. This DIEM trial shows that ovolipids intervention group had a significant increase in weight gain and decrease in waist circumference, compared to such changes in the dairy milk group (Table 2). Postprandial mean blood glucose, 1- hour after milk intake was also significantly lower in the ovolipids group compared to dairy milk group. Reduction in waist circumference, despite increase in BMI and body weight and less increase in blood glucose on supplementation of duck egg milk, is an interesting observation of our study. These markers are determinant of central obesity and hyperglycemia which are components of metabolic syndrome. The findings indicate that ovolipids duck egg milk, possibly, inhibit the development of metabolic syndrome among children getting inadequate breast milk and having undernutrition. It is known that wild type egg milk has low glycemic index and other benefits, due to high content of omega 3 fatty acids as well as essential and nonessential amino acids compared to dairy milk and milk powders [9]. Glycemic index of foods, is an attribute of carbohydrate-containing foods, however, many nutrients, may cluster, which favorably affect health.High glycemic index foods cause hyperglycemia which is known to cause damage to neurons, cardiomyocytes, vascular cells and beta cells of pancreas by: enhanced polyol activity, causing sorbitol and fructose accumulation; increased formation of advanced glycation end products; activation of protein kinase C and nuclear factor κB; and increased hexosamine pathway flux [12, 13]. Both acute and chronic hyperglycemic states can trigger all of these deleterious metabolic events through overproduction of superoxide anion and free fatty acids particularly in endothelial cells and beta cells of pancreas which can result in endothelial dysfunction leading to vascular damage and insulin resistance respectively.There is evidence that one banana increases blood glucose more than an apple that has the same amount of carbohydrate. Similarly, boiled sweet potato increases blood glucose more than boiled carrot [14-16]. Therefore, complete diets may be designed by selecting foods to have a desired overall glycemic index [14-16]. Some health agencies advocate consumption of low-glycemic index foods for prevention of metabolic syndrome, although the independent benefits of glycemic index or slowly absorbed foods are uncertain, particularly if people are already consuming a Mediterranean style diet rich in whole grains, vegetables, nuts, fruits and poultry. Our findings indicate that wild type egg, due to its low glycemic index and excellent tolerability, as well as due to its amino acids and fatty acids, could be an important addition to breast milk for children with or without undernutrition. There are no controlled trials on the effects of egg milk in babies on body weight and blood glucose, hence we can not compare our results with other studies. Such trials in infants and children are needed to control undernutrition and infection and to prevent childhood obesity and metabolic syndrome as well as CVDs and type 2 diabetes later in adult life. Randomized controlled trials of the effects of processed complementary foods among infants, aged 6-12 months, have shown a mixed impact on growth [17-19]. In three trials, the supplement increased weight and length; in two (including Indonesia) only weight was improved; and in another four (including Thailand) there was no effect on growth. However, in none of the study, children attained the expected growth velocity for age [17]. Systematic review and meta-analysis of home fortification of complementary foods, with products containing both micronutrients and a small amount of fat and protein, the pooled data from two efficacy trials in Africa suggest an effect size of ∼0.4 for both weight and height [18]. Supplementation of such foods also showed positive effects on indices of child development and morbidity in few studies. In a randomized, controlled intervention trial among 25 intervention and 26 control group babies, aged 6 months on breast feeding, DHA rich egg yolk was supplemented for 6 months. There was a significant increase in red blood cell DHA at 12 months of age in the intervention group along with significant improvement in visual evoked potential acuity (an index of maturation of retina and visual cortex) in the intervention group, compared to a decrease in DHA and no such visual acuity changes in the control group [20]. In an earlier study, among 8 nursing mothers, following consumption of omega-3 polyunsaturated fatty acid enriched eggs (two eggs daily) for 6 weeks, revealed a significant increase in long chain w-3 fatty acids in the breast milk indicating that a wild egg milk may simulate breast milk which could be important therapeutic approach for neural development among infants [21]. Clinical trials in adults that studied the effect of lowering glycemic index on insulin sensitivity and CVD risk factors reported diverse results [17-19, 22]. These findings may be related to concomitant changes in content of total carbohydrate and fiber as well as other protective nutrients; omega-3 fatty acids and polyphenolics, concomitant weight loss, and presence of and use of treatments for diabetes and CVDs. Complimentary feeding as well as circadian feeding should have a strong focus on nutrition as a child health issue which is framed in terms of individual health outcomes and immediate causes of undernutrition. (including stunting, wasting, low birth weight, maternal anemia, and exclusive breastfeeding) as well as obesity [19]. Nutrition may be described as the result of food intake, circadian food intake, chronotherapy with micronutrient intake, health status and care. This entails a focus on the proximal causes of child undernutrition as well as obesity. The focus of actions should be to intervention within the 1000 days, covering pregnancy and the first 2 years of life [17]. This approach may help in promoting, protecting and supporting breastfeeding as well as optimal nutrition of mothers, as a critical nutrition action, and on infant and young child feeding more broadly with complementary foods, possibly by circadian feeding. In both from developing and developed countries, breast milk from mothers with undernutrition or obesity, due to lack of energy as well as nutrients; flavonoids, omega-3 fatty acids, amino acids and other micronutrients may be inadequate for overall development of the infants, hence circadian feeding may be considered an important approach for optimal growth of children. Complimentary feeding and circadian feeding may also be used as an important method of intervention for health behavior change, to address behavioral, social, and environmental determinants of health in addition to delivering higher-quality care by the Centers for Medicare and Medical Services in United States and other countries [23, 24]. The Omni Carb Randomized Clinical Trial showed that adding DASH diet can modulate the adverse effects of high glycemic index on coronary risk factors and insulin resistance [24]. This interesting finding indicate that adding polyphenolics or fruit juice in the infant milk may provide more nutritious infant milk which may be protective against global burden of diseases [25]. It is possible that some of the deaths among premature newborn, possibly due to infection and entero-colitis may also be prevented by this infant milk [26].Further analysis of our data also shows that restricted feeding in the morning or evening revealed circadian stage dependent effects of milk at 4 weeks. The weight gain was statistically significant in both groups, whether intake was in the morning or evening (Ovolipid: AM. +0.50 kg, P = 0.017; PM. +0.40 kg, P = 0.002; Cow: AM. +0.25 kg, P = 0.013; PM. +0.27 kg, P = 0.004) (Figure 1). Corresponding changes in waist circumference were also significant (Ovolipid: AM. -2.2 cm, P = 0.040; PM. -1.7 cm, P = 0.038; Cow: AM. +1.0 cm, P = 0.044; PM. +2.4, P = 0.009). Since differences between AM and PM feeding were not statistically significant, results were pooled to compare changes associated with ovolipid vs, dairy milk. There was a significant difference in terms of weight gain (+0.45 vs. 0.26 kg, P = 0.027) and waist change (-2.0 vs. +1.7 cm, P <0.001) in the ovolipid group. It is possible that eating more foods in the morning may cause a decrease in body weight of the infants which may be protective among obese babies, whereas eating majority of the foods in the evening may cause gain in weight, which may be useful to prevent undernutrition [27-33]. The number of cases in this trail are too small to draw definite conclusions.
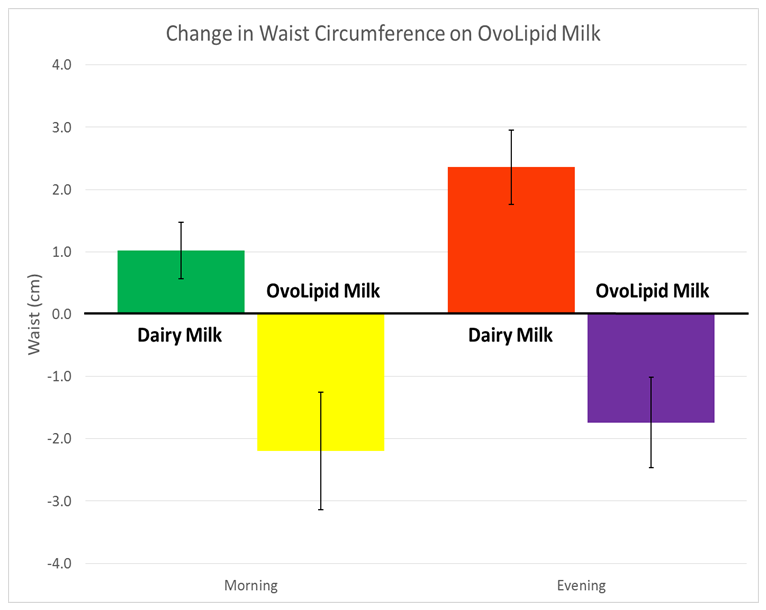 | Figure 1. Change in waist circumference on ovolipid milk and daily milk in morning and evening |
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML