Isichei-Ukah B. O., Imiere O. D.
Department of Microbiology, University of Benin, Benin City, Nigeria
Correspondence to: Isichei-Ukah B. O., Department of Microbiology, University of Benin, Benin City, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The study into the microbial quality of processed garri was carried out with the aim of assessing the impact of the processing environment on the microbial quality of the garri and also to determine if the amount of total sugar present in processed garri would contribute to its microbial load. The impact of the processing environment on the microbial quality of the garri was done by sample collection from possible sources of contamination from the processing environment, while the amount sugar present in the garri samples was determined using the phenol-sulfuric acid method. The result of the identification of the sources of contamination from the processing environment on the microbial quality of the garri showed that the processors are involved in practices such as unhygienic processing environment, citing of cassava effluent waste close to the processing site, poor sources of water supply, burying of basin inside the ground to serve as a discharged point for the grinded cassava paste from the machine and keeping of dried sack cassava paste on bare ground. These practices played a major role in the microbial contamination of the processed garri. The various stages were also found to harbour microorganisms such as Staphylococcus sp., Salmonella sp., Escherichia coli, Lactobacillus sp., Pseudomonas sp., Aspergillus niger, yeast cells, Penicillium chrysogenum and Penicillium notatum which directly or indirectly found its way to the processed garri. Microorganisms isolated from the different garri samples showed that microorganisms isolated from the identified sources of contamination apart from Mucor sp., Aspergillus fumigatus and Aspergillus tamari were all present in the garri. The results of the amount of the total sugar present in the garri samples did not show any relation with the microbial load. This study has showed that the cleanliness of the processing environment is a critical factor in the microbial quality of garri.
Keywords:
Garri, Processing, Environment, Quality, Microorganisms
Cite this paper: Isichei-Ukah B. O., Imiere O. D., Assessment of the Impact of Processing Environment on the Microbial Quality of Garri Processing, Food and Public Health, Vol. 7 No. 4, 2017, pp. 91-97. doi: 10.5923/j.fph.20170704.03.
1. Introduction
Garri is one of the staple locally processed foods in Nigeria. Its microbial quality depends on the management of each stage of its processing. It is taken in various forms in Nigeria, which include viscous paste (eba), soaking in cold water, mixed with other food like beans, moin-moin, etc. Garri itself may not constitute health hazard, since it has been estimated that various operation involved during processing usually resulted in reduction in its total cyanide content. The Garri quality can be defined on the basis of its safety and fitness for use by the target consumer. Thus in order to satisfy the taste of the consumers, a processor needs to integrate quality into the processing operations in order to build quality into the product. In so doing the processor is able to attract more customers and remain competitive in the market place. Both processors and consumers alike have various indices by which they judge the quality of garri. These include taste (acidity or sourness), swelling capacity, color, texture, crispiness, and absence of foreign matter (cleanliness). The product must not be too acidic, but should have a high swelling capacity, and must be of a definite color either white or cream. Sometimes the uniformity and brightness of the color is considered more important than the color itself (Arasi and Adebayo, 2000).With respect to texture, garri with a smooth texture is preferred. Garri must be crispy or very crispy and should have no sand particles, black specks, or residual peels in it.Unhygienic handling and poor sanitary measures that are very obvious has being observed between the last stage of production and the time its being displayed in the markets where the main patronage of many consumers could constitute serious health implications as many chances have been given to contamination by organisms of epidemiological importance (Arasi and Adebayo, 2000). Garri should be safe and suitable for human consumption, and free from abnormal flavors, odors, and living insects. Garri must be free from filth (impurities of animal origin, including dead insects) in amounts which may represent a hazard to human health.The main biological agents that contaminate garri are moulds, insects and mites (Ogiehor et al., 2005). Due to its richness in carbohydrate and other food substances, moulds such as Aspergillus, Penicillium, Fusarium, Rhizopus, Cladosporium and Mucor spp. are able to thrive on it especially during storage and distribution (Ekundayo, 1984). Aside it’s nutritional composition, practices such as processing and its handling methods such as drying on the floor, mat, rock, road side, etc, and even to the point of sale increases microbial contamination (Igiehor and Ikenehomeh, 2005). According to the authors, one of the major sources of contamination of the garri includes dust being raised by the breeze, storm, passing-by vehicle and every other form of air movement brings solid particles and heavy metals into garri. The effects of these contaminants could lead to the accumulation of some particles in the stomach, which can cause cancer and appendicitis. Dziedzoave et al. (2006), stated that the cassava peel, waste water from cassava processing operations, and other processing tools also constitute health hazards in the processing environment.Due to the economic importance of garri in human diet, this study is aimed to assess the impact of the processing environment on the microbial quality of processed garri and also to determine if the amount of sugar present in processed garri would contribute to its microbial load.
2. Materials and Methods
STUDY AREAThree study sites were purposely selected from Benin City, Edo state where white garri was processed. The sites sampled included Evoneka community in Ovia North local governemnet area, Ekosodin community Ovia North East local governement area and Isihor community. SAMPLE COLLECTIONThis was done according to the method described by Esiegbuya (2015) by collecting samples from the processing water, swap of the processing utensils and, working utensils soil samples, cassava effluents and airborne organisms.MICROBIOLOGICAL ANALYSIS Isolation of airborne microbesThe airborne microbes were isolated by exposing an already prepared potato dextrose agar and nutrient in a Petri dish at the processing environment for fifteen minutes, after which, the Petri dish was incubated under ambient temperature.Preparation of sterile water blankNine millilitres (99 ml) of distilled water was pipetted into twelve McCartney bottles representing each of the samples from the community village. The bottle was labeled S1 to S12. The bottles were the sterilized in an autoclave at 121°C for 15 minutes. After sterilization, 1g each of the sample representing each of the community was transferred into the McCartney bottles as stock solution.Serial dilutionMcCartney bottles divided into twelve groups labeled 101 to 106 were sterilized in an autoclave at 121°C for 15 minutes. With a sterile pipette, 1ml each was transferred from the stock bottles into the bottles labelled 101 to 106 containing 99 ml of sterilized distilled water for serial dilution preparation.Method of inoculation The pour plate method of inoculation technique was used in the isolation of the microorganisms associated with the different samples collected. One gram of each sample were aseptically weighed into 9 ml of 0.1% (w/v) sterile peptone water in a sterile McCartney bottles and allowed to stand for 5 min with occasional stirring using a magnetic stirrer. Thereafter, 10-fold serial dilutions of samples were made and 0.1 ml aliquot of each dilution was plated on potato dextrose agar (PDA, Biotec) supplemented with 0.1% concentrated lactic acid for total fungal counts and MacConkey agar (MA, Biotec) for coliform bacteria counts. The plates were incubated at 37°C for 24 h for bacteria and at 25°C for 3 to 5 days for fungi. After incubation, distinct colonies that developed were enumerated and expressed as colony-forming units per gram (cfu/g) of sample.MORPHOLOGICAL AND MICROSCOPIC IDENTIFICATION OF THE FUNGAL AND BACTERIAL ISOLATESThe isolated fungi were examined for characteristic macroscopic and microscopic features by comparison with the descriptions and illustrations of Barnett and Hunter (1998). The cultural characteristics such as the colony colour, margins, elevation and colony reverse colours were also recorded. Representative bacterial colonies obtained after incubation were purified by sub-culturing on nutrient agar using the streak plate method after which the purity of isolates was determined using the Gram stain. The purified isolates were then characterized and identified using their colonial, morphological and biochemical characteristics as described by Vanderzannt and Splittstoesser (1992) and Cheesbrough (2000) and with reference to the Bergey’s Manual of Determinative Bacteriology (Holt et al., 1994). For fungi, a wet mount preparation of the mycelial growth of each isolate was made using lactophenol cotton blue staining technique and observed under the microscope. The identification of the fungi was based on the examination of the conidial heads, philiades, conidiophore and the presence of foot cells or rhizoids (Barnett and Hunter, 1998).DETERMINATION OF pH, ACIDITY AND SUGAR CONTENT OF GARRI SAMPLES pHThe pH of each garri samples were determined using a reference electrode pH meter by homogenizing 10 g of each garri sample in 10 ml of sterile distilled water and the pH of the sample determined (Ogiehor and Ikenebomeh, 2005).Titratable acidity The Titratable acidity was determined as described by Friedrich (2001) using colorimetric acidity titration as follows: Equal parts of deionized distilled water (ddH2o) were added to the solid samples and macerated in a blenders at 100rpm for 2min. before centrifuging for 5min. at 2500rpm at room temperature. To 10ml of the supernatant sample solution in a clean Erlenmeyer flask were added 5 drops of 1% phenolphthalein indicator solution and a magnetic stir bar before stirring on a magnetic stir plate. Then 0.1N NaOH was carefully titrated against the sample solution to the end point of pH 8.2 until a faint but definite pink colour, which was stable for 5 to 10 seconds was obtained. The Titratable acidity was calculated using the equation: 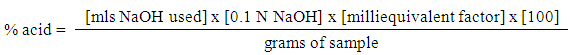 Where V is volume (ml) of NaoH solution used for titrationN = Normality of NaoH solution, meq.wt is milliequivalent weights of acid: lactic acid (0.067)Vs= sample volume The analysis was performed in triplicate to find the mean Titratable acidity of each sample. DETERMINATION OF SOLUBLE SUGAR Phenol solution25 g of phenol (Mallinckrodt, A.R.) was dissolved in 400 ml of water and diluted to make 500 ml of solution. Glucose stock solution10 mg of D glucose was dissolved in 80 ml of water and diluted to make 100 ml of stock solution (100 ug/ml).1ml aliquot of the above was pipetted into a test tube and 1ml water as blank was pipetted into another test tube. 1ml of 5% phenol solution was added and shaken followed by the careful addition of 5ml 96% H2SO4 to shake on a shaker vigorously for 2 min. and cooled. Standard glucose solutions of range 10-50µg/ml were treated with 5% phenol and H2SO4 as above. The absorbance of the golden yellow colour solution of the sample and glucose standard were read on a Spectronic 21D spectrophotometer at wave length 490nm against the blank. (AOAC 1990) the total soluble sugar was determined using the standard curve. Determination of total reducing sugarThis was done by dissolving 300g of sodium potassium tartrate in about 500 ml distilled water to form solution A. Solution B was prepared by dissolving 10 g of 3,5-dinitrosalicylic acid in 200 ml of 2N NaOH solution. The dinitrosalycilate reagent is prepared by mixing solutions A and B together and raising the final volume to 1 litre with distilled water.The regents was then prepared in duplicate by pipetting the reagents into a series of dry-clean and labelled test tubes. The tubes were then shaken well and place them in a boiling water bath for 5 minutes. After it has cooled the tubes 7.0 ml of distilled water was added and the optical density) of the colored solutions at 540 nm using a blank (control). The already prepared standard curve was used for the determination of the unknown concentration of the reducing sugar sample.
Where V is volume (ml) of NaoH solution used for titrationN = Normality of NaoH solution, meq.wt is milliequivalent weights of acid: lactic acid (0.067)Vs= sample volume The analysis was performed in triplicate to find the mean Titratable acidity of each sample. DETERMINATION OF SOLUBLE SUGAR Phenol solution25 g of phenol (Mallinckrodt, A.R.) was dissolved in 400 ml of water and diluted to make 500 ml of solution. Glucose stock solution10 mg of D glucose was dissolved in 80 ml of water and diluted to make 100 ml of stock solution (100 ug/ml).1ml aliquot of the above was pipetted into a test tube and 1ml water as blank was pipetted into another test tube. 1ml of 5% phenol solution was added and shaken followed by the careful addition of 5ml 96% H2SO4 to shake on a shaker vigorously for 2 min. and cooled. Standard glucose solutions of range 10-50µg/ml were treated with 5% phenol and H2SO4 as above. The absorbance of the golden yellow colour solution of the sample and glucose standard were read on a Spectronic 21D spectrophotometer at wave length 490nm against the blank. (AOAC 1990) the total soluble sugar was determined using the standard curve. Determination of total reducing sugarThis was done by dissolving 300g of sodium potassium tartrate in about 500 ml distilled water to form solution A. Solution B was prepared by dissolving 10 g of 3,5-dinitrosalicylic acid in 200 ml of 2N NaOH solution. The dinitrosalycilate reagent is prepared by mixing solutions A and B together and raising the final volume to 1 litre with distilled water.The regents was then prepared in duplicate by pipetting the reagents into a series of dry-clean and labelled test tubes. The tubes were then shaken well and place them in a boiling water bath for 5 minutes. After it has cooled the tubes 7.0 ml of distilled water was added and the optical density) of the colored solutions at 540 nm using a blank (control). The already prepared standard curve was used for the determination of the unknown concentration of the reducing sugar sample.
3. Results
1. Impact of the processing environment on the microbial quality of processed garri The practice of the garri processors in the three communities showed that the processors are involved in practices which contribute negatively to the microbial quality of the processed garri. Some of the practices includea. Burying of basin inside the ground to serve as a discharged point for the grinded cassava paste from the machine (Plate 1): This practice enhances soil particles and debris to fall directly into grinded paste thereby enhancing microbial contamination. The grinding machine was also found to be characterized by visibly unwashed left over paste. This serve as a source of contamination to fresh cassava paste because microorganisms such as Salmonella sp. E.coli, A. niger and P. chrysogenum isolated from the cassava paste collected from the grinding machine were also present in the processed garri. b. Keeping of dried sack cassava paste on bare ground. There is the possibility of soil microbes finding its way through the sack into the dried cassava paste. The microorganisms isolated from the dried cassava paste were also similar to that isolated from the soil samples (Table 1). Microorganisms isolated from the collected soil samples includes Pseudomonas sp, A.niger, Lactobacillus sp, Yeast cells and P. notatum.c. The floor of the manual presser was found to also have direct contact with ground also enhancing microorganisms contamination (Plate 3). d. Sitting of the cassava effluent site close to the processing sitee. Poor source of water. The microorganisms isolated from the water sources from the three communities were mainly Samonella sp and E. coli. f. Dirty processing environment. The processing environment was characterized by grasses, cassava waste and smell of effluent. The microorganisms isolated using the airborne method were mainly Staphyloccoccus sp and A, niger. | Plate 1. Cassava grinding machine with outlet directly expose to a ditch for cassava paste collection |
 | Plate 2. Dried cassava paste left on ground |
 | Plate 3. Floor of manual cassava paste press having direct contact with soil |
 | Plate 4. Cassava effluent dump site |
Table 1. Microbial count, total sugar, total reducing sugar and acidity of the processed garri samples
 |
| |
|
The overall results of the impact of the processing environment, processing water and grinding machine showed most of microorganisms isolated from theses sources were also present in the processed garri indicating the negative impact of the processing environment on the microbial quality of garri.The results of the mean bacterial and fungal count recorded on table 1 did not show any correlation to the total amount of sugar present in the garri samples. The result also showed that the amount of titratable acidity present in 10g of the garri samples collected from Evboneka, Ekosodin and Isihor communities was1.81, 0.67 and 0.67 respectively, while the amount of total sugar present in the garri samples was 0.99, 1.08 and 1.22mg for Evboneka, Ekosodin and Isihor communities The results of the microorganisms isolated from the different garri samples shown on Table 2 showed that Staphylococcus sp, E.coli, Salmonella sp Pseudomonas sp, Lactobacillus sp , A.niger, P. chrysogenum and A, niger were similar to those isolated from the working environment, soil samples, processing water, grinding machine and cassava effluent. This indicates that the garri samples might have been contaminated from these sources. The isolation of other microbes such as Mucor sp, A. fumigates and A. tamari might be from other sources of cross-contamination. Table 2. Microorganisms isolated from the working environment and working utensils
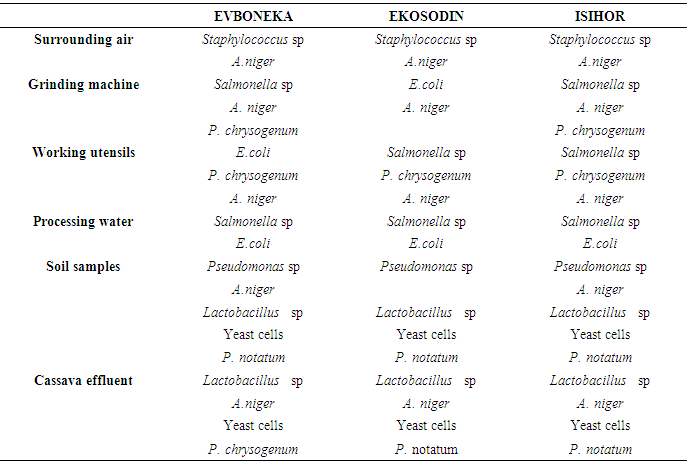 |
| |
|
Table 3. Microorganisms isolated from the different garri samples
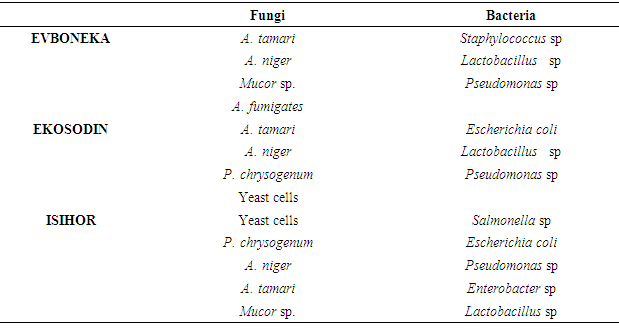 |
| |
|
Table 4. Cultural, microscopic and biochemical characteristics of the isolated bacteria
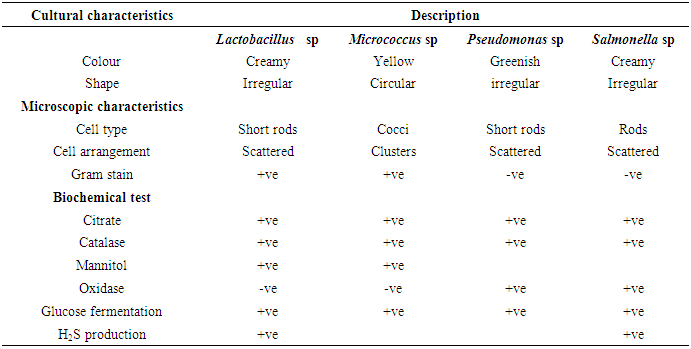 |
| |
|
Table 5. Fungi associated with soil samples collected during raining season
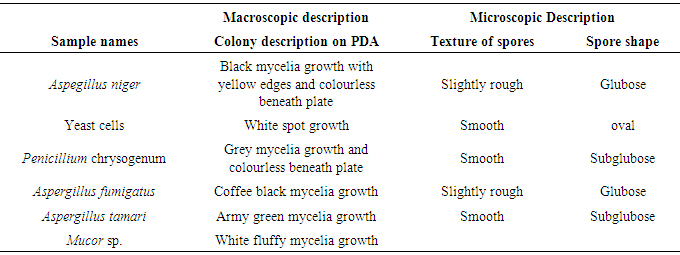 |
| |
|
4. Discussion
Garri is a product, obtained by fermenting peeled, washed and grated fresh cassava roots (for about 72 hours), dewatering and toasting (Ekwu and Ugwuona 2007). In Nigeria up to 70% of harvested cassava roots are processed into garri. Garri is normally consumed by adding water and sugar to taste and eaten as a refreshing snack drink or by pouring sufficient quantities in hot water to obtain a stiffen pudden (eba) (Onabolu et al, 2002,) which may be eaten with soup or stew. Garri is usually processed using manual processing method and mechanical processing method.Several authors have worked on the microbiological analyses of garri sold in market places. According to Adejumo et al. (2015) some of the microorganisms associated with garri sold in market places includes Bacillus cereus, Streptococcus sp., Escherichia coli, Aspergillus fumigatus and Fusarium sp. Orji et al. (2016) isolated pathogens such as Staphylococcus aureus, Escherichia coli and Streptococcus spp., Yersinia spp., Pseudomonas aeruginosa, Bacillus cereus, Escherichia coli and Staphylococcus aureus from garri. In this study, the microorganism isolated from the garri samples includes Staphylococcus sp., Escherichia coli, Salmonella sp., Pseudomonas sp., Lactobacillus sp., Aspergillus niger, Penicillium chrysogenum and Aspergillus niger, Mucor sp., Aspergillus fumigatus and Aspergillus tamari.The isolation of these organisms from the processed garri sample is an indication of microbial contamination of the different processing stages. According to Adejumo et al (2015), the microbial quality of garri depends on the management of each stage of processing and handling. The authors identified sources of microbial contamination of processed garri include the unhygienic handling and poor sanitary measures, methods of display in market places.In this study the identified sources of microbial contamination was mainly from the processing the processing environment. These include unhygienic processing environment, sitting of cassava effluent waste close to the processing site, poor sources of water supply, burying of basin inside the ground to serve as a discharged point for the grinded cassava paste from the machine and keeping of dried sack cassava paste on bare ground. These practices also played a major role in pollution of the processing environment which in turn affects the microbial quality of the garri. The findings in this study have shown that garri processors lack adequate knowledge on Sanitary and Phytosanitory Standards (SPS), Hazard Analysis and Critical Control Points (HACCP) and Good Processing Practices (GPP). This lack of knowledge in these areas is manifested was in the manner of their processing, personal hygiene and handling methods thus creating room for microbe contamination of the processed garri. According to Mensal et al. (2001) the problems of food safety in developed countries are different from those of developing countries whereas, in developing countries traditional methods of processing and packaging, improper holding temperature, poor personal hygiene of food handlers are still observed during food processing, packaging and marketing. Okolie, et al (2012) reports on the carbohydrate, pH, acidity and other functional properties of garri showed that the variation in the carbohydrate content in garri sample might be due to the processing operations while the low acid values were due to the activities of microorganisms which hydrolyzes starch to organic acids (mainly lactic acid) known to be responsible for the acidic nature of garri.In this study the total carbohydrate content was found to vary among the collected garri samples and the amount of total sugar did not enhance the microbial load of the processed garri but may serves as a source of nutrient for the microorganism to thrive.
5. Conclusions
Due to economic importance of garri in human diet, there is the need for government especially in developing countries where garri is processed to put policies in place that will ensure that food processors (garri processors) be trained on the importance of personal hygiene and Good Processing Practices (GPP).
References
| [1] | Adejumo, A. O. D., Adebayo G. G. and Komolafe, C. A. (2015). Solid and Microbiological Quality Assessment of Gari within Ibadan Metropolis Journal of Scientific Research and Reports, 4(2): 162-167. |
| [2] | AOAC., (2004) (Official Methods of Analysis (16th edition). Association of Official Analytical Chemists, Washington DC. |
| [3] | Arasi, M.A and Adebayo G.G. (2000). Survey of microbial pathogen in Gari Displayed in open markets within Ibadan Metropilis. Proceedings of the 18th Annual Conference of the Nigerian Institute of Science and Technology. 2000; 83–93. |
| [4] | Barnett H. L. and Hunter, B. B. (1998). Descriptions and Illustrations of genera. Illustrated Genera of Imperfect fungi. The American Phytopathological Society press. St. Paul Minnesota.59-218p. |
| [5] | Cheesbrough, M. (2000). District laboratory practice in tropical countries Part 2. Cambridge: Cambridge University Press, London. |
| [6] | Dziedzoave, N.T., Abass A.B., Amoa-Awua, W. K. A. and Sablah. M. (2006). Quality management manual for the production of high quality cassava flour, edited by G.O. Adegoke and L. Brimer. International Institute of Tropical Agriculture, Ibadan. 68 p. |
| [7] | Ekundayo, C. A. (1984). Microbial spoilage of packaged garri in storage. Microbiological Letters 23: 271-278. |
| [8] | Ekwu F.C. and Ugwuona, S. (2007). Physiochemical and sensory properties of garri from cassava food during short term storage. International Journal of Food Science and dehydrated cassava chips. Journal of Science of Agriculture, Food Technology and Environment. 44-48. |
| [9] | Friedrich, J.E. (2001). Titrable activity of acid tastants. Current Protocols in Food Analytical Chemistry. John Wiley & Sons, Inc.,G2.1.1 – G. 2.1.7. |
| [10] | Holt, J. G., Krieg, N. R., Sneath, P. H. A., Staley, J. T., and Williams, S. T. (1994). Bergey’s Manual of Determinative Bacteriology, 9th edn. Lippincott Williams and Wilkins, Baltimore, MD. |
| [11] | Mensah, S. A. (2001): Energy for rural women’s enterprises - Ghana. Economic empowerment of women in Africa, 37-43p. |
| [12] | Ogiehor, I. S, Ikenebomeh, M. J. and Ekundayo, A. O. (2007). The bioload and aflatoxin content of market Garri from some selected states in Southern Nigeria. African Health Science 7(4): 223-227. |
| [13] | Ogiehor, I. S., and Ikenebomeh, M. J. (2005). Extension of shelf life of garri by hygienic handling and sodium benzoate treatment. African Journal of Biotechnology 4: 744 - 748. |
| [14] | Okolie, N. P., Brai, M. N. and Atoyebi, O. M. (2012). Comparative Study on Some Selected Garri Samples Sold in Lagos Metropolis Journal of Food Studies 1(1) 33-42. |
| [15] | Onabolu, A.O., Oluwole, O. S. and bokanga, M. (2002). Loss of Residual Cyanogens in a cassava food during short-term storage. International Journal of Food Sciences and Nutrition 52: 343–349. |
| [16] | Orji, J.O., Nnachi, A.U., Ojiochie, C.O., Egwuatu, C.C., Efunshile, A.M., Ezejiofor, O.I., Ezeama, C.O., Nwaneli, C.U. and Mbachu, I. (2016). International journal of scientific & technology research 5(11) 85-91. |
| [17] | Vanderzannt, C. and Splittstoesser, D. F. (1997). Compendium of methods for the microbiological examination of foods, 3rd ed. American Public Health Association, Washington DC, 596p. |



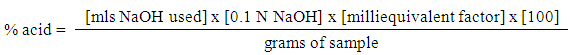 Where V is volume (ml) of NaoH solution used for titrationN = Normality of NaoH solution, meq.wt is milliequivalent weights of acid: lactic acid (0.067)Vs= sample volume The analysis was performed in triplicate to find the mean Titratable acidity of each sample. DETERMINATION OF SOLUBLE SUGAR Phenol solution25 g of phenol (Mallinckrodt, A.R.) was dissolved in 400 ml of water and diluted to make 500 ml of solution. Glucose stock solution10 mg of D glucose was dissolved in 80 ml of water and diluted to make 100 ml of stock solution (100 ug/ml).1ml aliquot of the above was pipetted into a test tube and 1ml water as blank was pipetted into another test tube. 1ml of 5% phenol solution was added and shaken followed by the careful addition of 5ml 96% H2SO4 to shake on a shaker vigorously for 2 min. and cooled. Standard glucose solutions of range 10-50µg/ml were treated with 5% phenol and H2SO4 as above. The absorbance of the golden yellow colour solution of the sample and glucose standard were read on a Spectronic 21D spectrophotometer at wave length 490nm against the blank. (AOAC 1990) the total soluble sugar was determined using the standard curve. Determination of total reducing sugarThis was done by dissolving 300g of sodium potassium tartrate in about 500 ml distilled water to form solution A. Solution B was prepared by dissolving 10 g of 3,5-dinitrosalicylic acid in 200 ml of 2N NaOH solution. The dinitrosalycilate reagent is prepared by mixing solutions A and B together and raising the final volume to 1 litre with distilled water.The regents was then prepared in duplicate by pipetting the reagents into a series of dry-clean and labelled test tubes. The tubes were then shaken well and place them in a boiling water bath for 5 minutes. After it has cooled the tubes 7.0 ml of distilled water was added and the optical density) of the colored solutions at 540 nm using a blank (control). The already prepared standard curve was used for the determination of the unknown concentration of the reducing sugar sample.
Where V is volume (ml) of NaoH solution used for titrationN = Normality of NaoH solution, meq.wt is milliequivalent weights of acid: lactic acid (0.067)Vs= sample volume The analysis was performed in triplicate to find the mean Titratable acidity of each sample. DETERMINATION OF SOLUBLE SUGAR Phenol solution25 g of phenol (Mallinckrodt, A.R.) was dissolved in 400 ml of water and diluted to make 500 ml of solution. Glucose stock solution10 mg of D glucose was dissolved in 80 ml of water and diluted to make 100 ml of stock solution (100 ug/ml).1ml aliquot of the above was pipetted into a test tube and 1ml water as blank was pipetted into another test tube. 1ml of 5% phenol solution was added and shaken followed by the careful addition of 5ml 96% H2SO4 to shake on a shaker vigorously for 2 min. and cooled. Standard glucose solutions of range 10-50µg/ml were treated with 5% phenol and H2SO4 as above. The absorbance of the golden yellow colour solution of the sample and glucose standard were read on a Spectronic 21D spectrophotometer at wave length 490nm against the blank. (AOAC 1990) the total soluble sugar was determined using the standard curve. Determination of total reducing sugarThis was done by dissolving 300g of sodium potassium tartrate in about 500 ml distilled water to form solution A. Solution B was prepared by dissolving 10 g of 3,5-dinitrosalicylic acid in 200 ml of 2N NaOH solution. The dinitrosalycilate reagent is prepared by mixing solutions A and B together and raising the final volume to 1 litre with distilled water.The regents was then prepared in duplicate by pipetting the reagents into a series of dry-clean and labelled test tubes. The tubes were then shaken well and place them in a boiling water bath for 5 minutes. After it has cooled the tubes 7.0 ml of distilled water was added and the optical density) of the colored solutions at 540 nm using a blank (control). The already prepared standard curve was used for the determination of the unknown concentration of the reducing sugar sample.



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML



