-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2017; 7(4): 75-80
doi:10.5923/j.fph.20170704.01

A Comparative Study to Evaluate the Polyphenol Content and Antioxidant Activity of Coffee with Fresh Whole Milk and Evaporated Milk
Hadeil M. Alsufiani, Ulfat M. Omar
Biochemistry department, faculty of sciences, King Abdulaziz University, Jeddah, Saudi Arabia
Correspondence to: Hadeil M. Alsufiani, Biochemistry department, faculty of sciences, King Abdulaziz University, Jeddah, Saudi Arabia.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In Saudi Arabia, consumption of coffee with milk either fresh whole milk or evaporated milk is a regular habit of a daily life. Evaporated milk differs from fresh whole milk in containing approximately more than twice the amount of solids. These solids such as fat and protein were reported to affect the coffee antioxidants activity. Thus, the aim of this study was to compare the effect of adding evaporated and fresh milk on the total phenolic content, total flavonoids content and the antioxidant capacity of coffee. Total phenolic and total flavonoids content, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and Hydrogen peroxide (H2O2) Radical Scavenging Activity, Reducing power and Ferrous Ion Chelating Activity (FIC) were measured. The results showed that the addition of evaporated milk to coffee reduces the hydrogen peroxide and DPPH scavenging activity of coffee antioxidant compared with fresh milk. In contrast, evaporated milk enhances the reducing power and metal chelation of the coffee than fresh milk. In conclusion, adding fresh whole milk or evaporated milk to coffee influences its antioxidant properties by different mechanisms.
Keywords: Coffee, Evaporated milk, Whole fresh milk, Antioxidants activity, Polyphenols, Scavenging activity
Cite this paper: Hadeil M. Alsufiani, Ulfat M. Omar, A Comparative Study to Evaluate the Polyphenol Content and Antioxidant Activity of Coffee with Fresh Whole Milk and Evaporated Milk, Food and Public Health, Vol. 7 No. 4, 2017, pp. 75-80. doi: 10.5923/j.fph.20170704.01.
Article Outline
1. Introduction
- Coffee is one of the most commonly consumed beverages worldwide [1]. Coming from the roasted seeds of several species of the genus Coffea, the popularity of coffee is due to its aroma, flavor and caffeine content which plays a major role in its popularity. Coffee is a complex mixture of more than a thousand different chemicals, many of which are reported to be biologically active compound [2, 3]. Of these compounds are polyphenols which exhibit strong antioxidant activity [4]. Several epidemiological studies reported that coffee antioxidants could exert protective effect against some cardiovascular risk factors [5] and some types of cancer [6].In Saudi Arabia, consumption of coffee with milk either fresh whole milk or evaporated milk is a regular habit of a daily life. The most commonly consumed fresh whole milk brand in the gulf countries contains 3.5g protein, 3.0 g fat, 5.5 g carbohydrates, 120 mg calcium and 91 mg phosphorus per 100 g of milk. On the other hand, evaporated milk contains 7g protein, 7.9 g fat, 9.7 g carbohydrates, 252 mg calcium and 205 mg phosphorus per 100 g of milk [7]. Thus, Evaporated milk differs from fresh whole milk in containing approximately more than twice the amount of solids. These solids such as fat and protein were reported to affect the coffee antioxidants activity due to the formation of polyphenol-protein complexes [8-10]. Based on this, we hypothise that the different solid content between fresh whole milk and evaporated milk will affect the antioxidant properties of coffee. Therefore, the aim of this study is to compare the effect of adding evaporated and fresh milk on the total phenolic content, total flavonoids content and the antioxidant capacity of coffee.
2. Materials and Methods
2.1. Preparation of Coffee Samples
- Red mug coffee (Nescafé), fresh whole milk and evaporated milk were purchased from Saudi local market. The coffee with milk samples were prepared as Saudi people commonly drink it. Two grams of red mug coffee was dissolved in 200 ml boiling water. Then, 10 ml, 40 ml and 60 ml of fresh or evaporated milk were added to have a final concentration of 10%, 20% and 30% milk, respectively. All the experiments were done at the biochemistry department labs, faculty of science, King AbdulAziz University in the period from September 2016 until February 2017.
2.2. Total Phenolic and Total Flavonoids Content
- The total phenolic content of the coffee samples was determined by a modified Folin-ciocalteu’s assay [11]. Half ml of each coffee sample (with fresh or evaporated milk) were added in a test tube. After that, 5 ml of deionized water and 5 ml of Folin-Ciocalteu’s reagent (DBH, Poole, England) were added to each coffee sample and incubated at room temperature for 5 minutes. Then, 1 ml of 2% anhydrous sodium carbonate (CDH, New Delhi, India) was added to the samples and incubated in dark place for one hour. After incubation, the optical density of the coffee samples was measured at wavelength of 750 nm by spectrophotometer. The experiment was repeated 3 times for each sample and the average optical density was calculated. The total phenolic content of the coffee samples was then determined by comparing the average optical density of the samples to the standard curve. Different concentrations of gallic acid (0 -1000 µg/ml in 50% methanol) (Sigma-Aldrich, Poole, UK) were used as standards and the results were expressed as µg of gallic acid equivalents/ 2 g of coffee). The total flavonoids content of the coffee samples was determined by Aluminum chloride colorimetric method [12]. Two hundred and fifty microliters of each coffee sample (with fresh or evaporated milk) were added in a test tube. Then, 1.25 ml of deionized water and 75 µl of sodium nitrate (Sigma-Aldrich, Poole, UK) were added to the samples and incubated at room temperature for 5 minutes. After incubation, 10% aluminium chloride (loba chemie PVT.LTD., Mumbai, India) (150 µl), 1M sodium hydroxide (AppliChem Panreac, Missouri, USA) (0.5 ml) and deionized water (275 µl) were added to the test tubes. The optical density of the color formed was measured spectrophotometrically at 510 nm. The experiment was repeated 3 times for each sample and the average optical density was calculated. The total flavonoids content of the coffee samples was then determined by comparing the average optical density of the samples to the standard curve. Different concentrations of catechin (0 - 500 µg/ml) (Sigma-Aldrich, Poole, UK) were used as standards and the results were expressed as µg of catechin / 2 g of coffee).
2.3. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) and Hydrogen Peroxide (H2O2) Radical Scavenging Activity
- The free radical scavenging activity of the stable DPPH by coffee samples with both types of milk were determined by a modified Blois method (1958) [13]. Each coffee sample (250 µl) were diluted in 99.5% ethanol (Sigma-Aldrich, Poole, UK) and added to 62.5 µl of DPPH (0.02%) (Sigma-Aldrich, Poole, UK). Then, the samples were shacked vigorously and incubated in dark place for one hour. Finally, the optical density of the samples was measured at wavelength of 517 nm by spectrophotometer. The experiment was repeated 3 times for each sample and the average optical density was calculated. The percentage of DPPH scavenging activity was then calculated by using the following equation: % inhibition= [ (ODCONTROL- ODSAMPLE)/ ODCONTROL ] x 100, where OD is optical density.The efficiency of antioxidants in coffee samples (with fresh and evaporated milk) to scavenge hydrogen peroxide was determined by Ruch et al (1989) method [14]. Briefly, hydrogen peroxide solution (40 Mm) (Sigma-Aldrich, Poole, UK) was prepared in phosphate buffer at pH 7.4. Then, 0.6 ml of this solution was added to 1ml of each sample and incubated at room temperature for 10 minutes. After that, the optical density of the samples was measured by spectrophotometer at wavelength of 517 nm. The experiment was repeated 3 times for each sample and the average optical density was calculated. The percentage of H2O2 scavenging activity was then calculated by using the following equation: % inhibition= [(ODCONTROL- ODSAMPLE)/ ODCONTROL] x 100, where OD is optical density.
2.4. Reducing Power and Ferrous Ion Chelating Activity (FIC)
- Oyaizu method (1986) was used to estimate the reducing power of the coffee samples with fresh and evaporated milk [15]. First, phosphate buffer (1.25 ml) (0.2 M, pH 6.6) (Oxoid, Hampshire, England) was mixed with each coffee sample (1 ml) and ferric cyanide (1.25 ml) (1%) (Koch-Light, Colnbrook Bucks, UK). Then, the samples were incubated for 30 minutes at 50°C before the trichloroacetic acid (TCA) (1.25 ml) (10%) (Sigma-Aldrich, Poole, UK) was added to the mixture to stop the reaction. After that, the samples were centrifuged for 10 minutes at 6000 rpm and the supernatant was then diluted with equal amount of distilled water and ferric chloride (1%) (BDH, Poole, England). Last, the optical density of the samples was measured immediately at wavelength of 700 nm by spectrophotometer. The experiment was repeated 3 times for each sample and the average optical density was calculated. Optical density results were then used to indicate the reducing power of the coffee antioxidants. Ferrous ion chelation of the coffee samples with fresh and evaporated milk was determined by Dinis et al method (1994) [16]. Five microliters of each coffee sample was incubated at room temperature for 30 seconds with 1.5 ml of distilled water and 50 µl of 2mM ferrous chloride (Sigma-Aldrich, Poole, UK). After the first incubation, all samples were then incubated for a second time with 100 µl of ferrozine (Sigma-Aldrich, Poole, UK) for 10 minutes. Finally, the optical density of the samples was measured immediately at wavelength of 562 nm by spectrophotometer. The experiment was repeated 3 times for each sample and the average optical density was calculated. The percentage of chelating activity was then calculated by using the following equation: % chelating activity= [(ODCONTROL- ODSAMPLE)/ ODCONTROL] x 100, where OD is optical density.
2.5. Statistical Analysis
- To test the difference in total polyphenols and total flavonoids between coffee with evaporated milk and coffee with fresh milk, independent t-test was used. The same approach was used to assess the differences in antioxidant capacity between coffee with evaporated milk and coffee with fresh milk. All statistical analysis was performed using the statistical software GraphPad prism 7. Data were presented as means ± SD and the statistical significance was taken as P < 0.05.
3. Results
3.1. Total Phenolic and Total Flavonoids Content
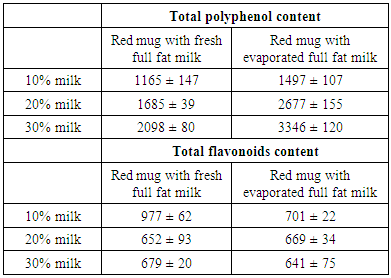
- Table 1 shows the mean total polyphenol and flavonoids content estimated by Folin-Ciocalteu and aluminium chloride assay, respectively in red mug coffee with fresh and evaporated milk. Content of total polyphenols in red mug coffee with evaporated full fat milk using different concentrations (10%, 20% and 30%) were higher than in red mug coffee with fresh whole milk but did not differ significantly. similarly, there were no significant difference in total flavonoids content between red mug coffee with fresh milk and red mug coffee with evaporated milk.
3.2. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) and Hydrogen Peroxide (H2O2) Radical Scavenging Activity
- The radical scavenging activity of antioxidants in red mug coffee with fresh whole milk or full fat evaporated milk was expressed as inhibition percentage (Figure 1). The results showed that the percentage of inhibition of DPPH by red mug with 10% evaporated full fat milk was significantly lower compared with red mug with fresh whole milk. Similarly, red mug with 30% evaporated milk showed a significant lower percentage of inhibition of DPPH than in red mug with fresh whole milk.
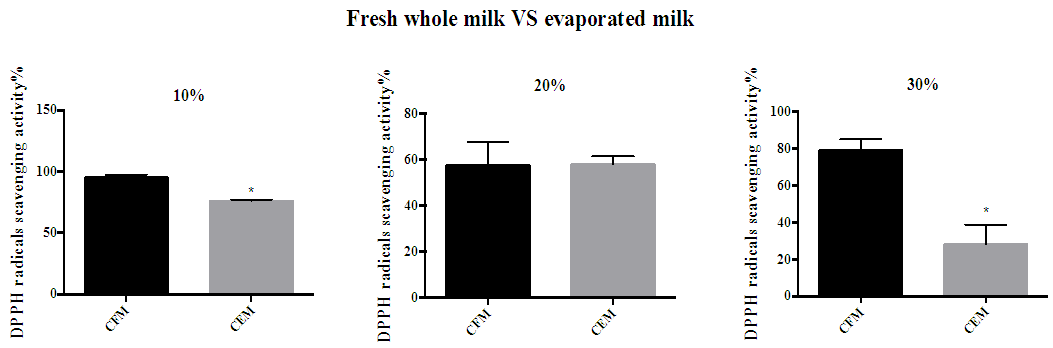 | Figure 1. Percentage of DPPH inhibition by antioxidants in red mug coffee with fresh and evaporated milk. (A) 10% full fat milk (B) 20% full fat milk (C) 30% full fat milk. Error bars shows SD. * P<0.05 when compared with red mug coffee with fresh whole milk. CFM; cow’s fresh milk. CEM; cow’s evaporated milk |
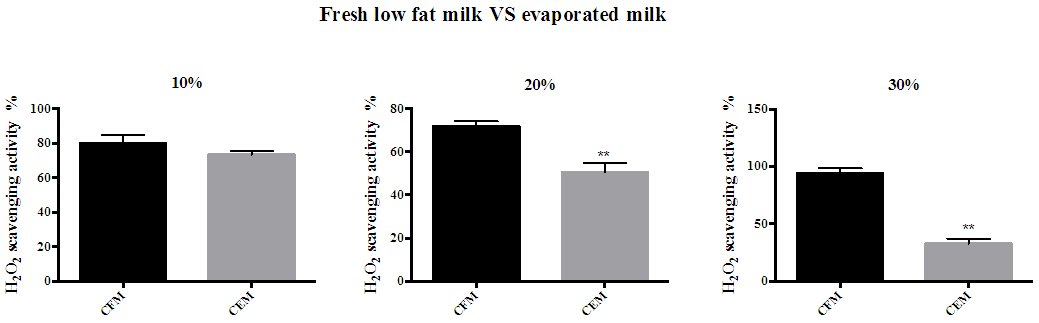 | Figure 2. Hydrogen peroxide scavenging activity of red mug coffee fresh whole milk and evaporated full fat milk. (A) 10% full fat milk (B) 20% full fat milk (C) 30% full fat milk. Error bars shows SD. ** P<0.01 when compared with red mug coffee with fresh whole milk. CFM; cow’s fresh milk. CEM; cow’s evaporated milk |
3.3. Reducing Power and Ferrous Ion Chelating Activity (FIC)
- Figure 3 shows the reducing power activity of red mug coffee with fresh whole milk and evaporated milk. Red mug coffee with either 20% or 30% evaporated milk had a significantly higher reductive ability than red mug coffee with fresh milk.
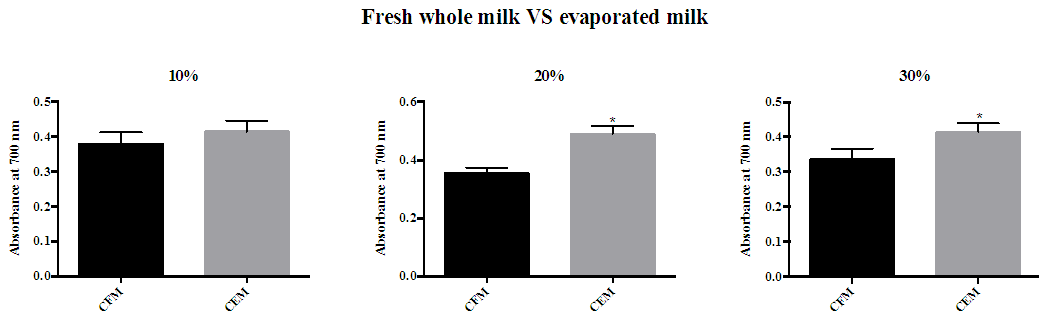 | Figure 3. Reducing power of red mug coffee with fresh and evaporated milk. (A) 10% full fat milk (B) 20% full fat milk (C) 30% full fat milk. Error bars shows SD. * P<0.05 when compared with red mug coffee with fresh whole milk. CFM; cow’s fresh milk. CEM; cow’s evaporated milk |
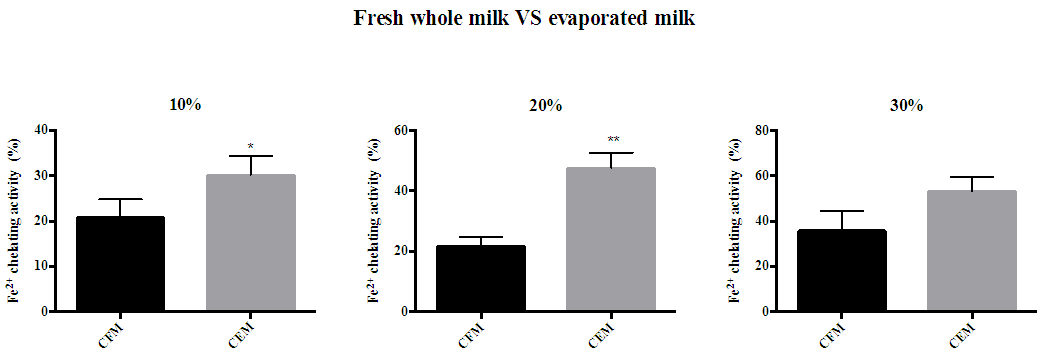 | Figure 4. Ferrous chelating activity of red mug coffee with fresh and evaporated milk. (A) 10% full fat milk (B) 20% full fat milk (C) 30% full fat milk (D). Error bars shows SD. * P<0.05 and ** P<0.01 when compared with red mug coffee with fresh whole milk. CFM; cow’s fresh milk. CEM; cow’s evaporated milk |
4. Discussion
- In this study, the total phenolic content and the antioxidant capacity of red mug coffee with fresh whole milk and evaporated milk were determined. The antioxidant capacity was measured by using four different methods including DPPH radical scavenging activity, hydrogen peroxide radical scavenging activity, reducing power and ferrous ion chelating activity.
4.1. Total Phenolic and Total Flavonoids Content
- Several studies reported that the addition of fresh or evaporated milk to coffee increased the total polyphenol and flavonoids content of coffee [8, 10]. This is could be due to the presence of other compounds in milk such as lactoferrin, ascorbic acid, tocopherol and tocotrienols that can act as antioxidants [17]. We assumed that fresh milk and evaporated milk contains the same amount of these compounds. Thus, our results showed no significant difference between red mug coffee with fresh milk and coffee with evaporated milk in total polyphenol and total flavonoids content.
4.2. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) and Hydrogen peroxide (H2O2) Radical Scavenging Activity
- To determine the scavenging activity of coffee samples with fresh whole milk and evaporated milk, two assays were used which are DPPH and H2O2 radical scavenging activity assays. In the DPPH scavenging activity, the assay is depend on the reduction of DPPH by hydrogen donating substances such as polyphenols and flavonoids into DPPH-H resulting in changing the color of the solution from purple to yellow [18, 19]. On the other hand, the H2O2 scavenging assay is based on the donation of electrons by the phenolic groups of polyphenols to neutralize hydrogen peroxide into water [20]. In the present study, the addition of evaporated milk to coffee reduces the hydrogen peroxide and DPPH scavenging activity of coffee antioxidant compared with fresh milk. This could be explained by the higher content of protein and fats in evaporated milk than fresh whole milk. Casein and whey are the major types of protein in milk account for 80% and 20% [21]. Several studies found that polyphenols can bind casein and ß-lactoglobulin (a major whey protein) via both hydrophilic and hydrophobic interaction forming protein-polyphenol complexes. These interactions could be either multidentate interactions (one polyphenol bound on multiple sites of one or many proteins) or multi-site (many polyphenols bound to one protein). Further stabilization of this complex is suggested to be due to the formation of hydrogen bonds between the amino acid residues. The binding of polyphenols with proteins can affect the electron donation of polyphenols by reducing the numbers of OH groups available in the solution. Furthermore, the presence of high amounts of lipids in the evaporated milk could interact with milk proteins results in masking the radical scavenging activity of polyphenol [9, 22-27].
4.3. Reducing Power and Ferrous Ion Chelating Activity (FIC)
- Reducing power and ferrous ion chelating activity assays were used to determine the ability of antioxidants in coffee with milk (both evaporated and fresh) to act as a metal chelator and as a reducing agent. In the reducing power assay, the method is based on the reduction of ferric ions (Fe3+) to ferrous ion (Fe2+). Potassium ferricyanide in the presence of reducing agents such as antioxidants will be converted to potassium Ferrocyanide, which then can react with ferric chloride forming ferric-ferrous complex. Thus, this method gives a good indication of antioxidants that donates electrons [28-30]. In ferric ion chelation assay, the principle of the method is depend on the formation of the ferrous-ferrozine complex (red color). When the reaction contains antioxidants, the formation of this complex is inhibited due to the ability of antioxidants to chelate iron ions resulting in the decrease of the optical density of the complex [31, 32]. In our study, coffee with evaporated milk showed a higher reducing power and metal chelation than fresh whole milk. As mentioned earlier, evaporated milk contains double the amount of protein compared with fresh whole milk. These results is in concordance with wipatanawin et al (2015) results who found that the addition of higher protein milk content promotes the metal chelating activity of Oolong tea than the addition of lower protein milk content [33]. Furthermore, chiang and chang (2005) reported that both casein and whey protein had metal chelating activity which directly increased with the concentration of milk [34]. These findings could explain why the addition of evaporated milk to coffee increased the metal chelation activity of the samples compared with fresh whole milk.
5. Conclusions
- In conclusion, the addition of evaporated milk to coffee reduces the hydrogen peroxide and DPPH scavenging activity of coffee antioxidant compared with fresh milk. conversely, evaporated milk enhances the reducing power and metal chelation of the coffee than fresh milk. Therefore, adding fresh whole milk or evaporated milk to coffee influences its antioxidant properties by different mechanisms.
References
| [1] | Grigg, D., The worlds of tea and coffee: Patterns of consumption. GeoJournal, 2002. 57(4): p. 283-294. |
| [2] | Butt, M.S. and M.T. Sultan, Coffee and its Consumption: Benefits and Risks. Critical Reviews in Food Science and Nutrition, 2011. 51(4): p. 363-373. |
| [3] | Godos, J., et al., Coffee components and cardiovascular risk: beneficial and detrimental effects. International Journal of Food Sciences and Nutrition, 2014. 65(8): p. 925-936. |
| [4] | Spiller, M.A., The chemical components of coffee. Prog Clin Biol Res, 1984. 158: p. 91-147. |
| [5] | Miranda, A.M., et al., Association between Coffee Consumption and Its Polyphenols with Cardiovascular Risk Factors: A Population-Based Study. Nutrients, 2017. 9(3). |
| [6] | Nkondjock, A., Coffee consumption and the risk of cancer: an overview. Cancer Lett, 2009. 277(2): p. 121-5. |
| [7] | Musaiger, A.O., food composition tables for arab gulf countries (gulfoods). 2006: arab center for nutrition. |
| [8] | Al-Ghafari, A., et al., the effect of adding different concentrations of cow;s milk on the antioxidant properties of coffee. biosciences biotechnology research asia, 2017. 14(1): p. 177-184. |
| [9] | Yuksel, Z., E. Avci, and Y.K. Erdem, Characterization of binding interactions between green tea flavanoids and milk proteins. Food Chemistry, 2010. 121: p. 450-456. |
| [10] | Al Doghaither, H., et al., The addition of evaporated milk alters the antioxidant properties of the most commonly consumed coffee in saudi arabia. Malaysia Applied Biology Journal, 2017. 46(2): p. 1-8. |
| [11] | Singleton, V.l., R. Orthofer, and R.M. Lamuela-Raventos, Analysis of total phenols and other substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology, 1999. 299: p. 152-178. |
| [12] | Ayuko, T.A.a., et al., In vitro antiplasmodial activity and toxicity assessment of plant extracts used in traditional malaria therapy in the Lake Victoria Region. Memórias do Instituto Oswaldo Cruz, 2009. 104: p. 689-694. |
| [13] | Blois, M.S., Antioxidant Determinations by the Use of a Stable Free Radical. Nature, 1958. 181(4617): p. 1199-1200. |
| [14] | Ruch, R.J., S.J. Cheng, and J.E. Klaunig, Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis, 1989. 10(6): p. 1003-8. |
| [15] | Oyaizu, M., Studies on Products of Browning Reaction Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. The Japanese Journal of Nutrition and Dietetics, 1986. 44(6): p. 307-315. |
| [16] | Dinis, T.C., V.M. Maderia, and L.M. Almeida, Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys, 1994. 315(1): p. 161-9. |
| [17] | Lindmark-Mansson, H. and B. Akesson, Antioxidative factors in milk. Br J Nutr, 2000. 84 Suppl 1: p. S103-10. |
| [18] | Alam, M.N., N.J. Bristi, and M. Rafiquzzaman, Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharmaceutical Journal, 2013. 21(2): p. 143-152. |
| [19] | Poonia, P., et al., In-vitro antioxidant potential of Jasminum mensyi Hance (leaves) extracts. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2011. 2(1): p. 348-357. |
| [20] | Ebrahimzadeh, M.A., S.F. Nabavi, and S.M. Nabavi, Antioxidant activities of methanol extract of Sambucus ebulus L. flower. Pak J Biol Sci, 2009. 12(5): p. 447-50. |
| [21] | Jianhui, Y., et al., Interactions of black and green tea polyphenols with whole milk. Food Research International, 2013. 53(1): p. 449-455. |
| [22] | Hasni, I., et al., Interaction of milk α- and β-caseins with tea polyphenols. Food Chemistry, 2011. 126: p. 630-639. |
| [23] | O'Connell, J.E. and P.F. Fox, Proposed mechanism for the effect of polyphenols on the heat stability of milk. International Dairy Journal, 1999. 9(8): p. 523-536. |
| [24] | Prigent, S.V., et al., Effects of non-covalent interactions with 5-O-caffeoylquinic acid (chlorogenic acid) on the heat denaturation and solubility of globular proteins. J Agric Food Chem, 2003. 51(17): p. 5088-95. |
| [25] | Kanakis, C.D., et al., Milk β-lactoglobulin complexes with tea polyphenols. Food Chemistry, 2011. 127(3): p. 1046-1055. |
| [26] | Sharma, V., H. Vijay Kumar, and L. Jagan Mohan Rao, Influence of milk and sugar on antioxidant potential of black tea. Food Research International, 2008. 41(2): p. 124-129. |
| [27] | Arts, M.J., et al., Interactions between flavonoids and proteins: effect on the total antioxidant capacity. J Agric Food Chem, 2002. 50(5): p. 1184-7. |
| [28] | Jayanthi, P. and P. Lalitha, Reducing power of the solvent extracts of Eichhornia crassipes (Mart.) Solms International Journal of Pharmacy and Pharmaceutical Sciences, 2011. 3(3): p. 126-128. |
| [29] | Bolanos de la Torre, A.A., et al., A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem, 2015. 174: p. 119-23. |
| [30] | Yildirim, A., A. Mavi, and A.A. Kara, Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem, 2001. 49(8): p. 4083-9. |
| [31] | Geckil, H., et al., Antioxidant, Free Radical Scavenging and Metal Chelating Characteristics of Propolis. American Journal of Biochemistry and Biotechnology, 2005. 1(1): p. 27-31. |
| [32] | Chanda, S. and R. Dave, In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: An overview. African Journal of Microbiology Research, 2009. 3(13): p. 981-996. |
| [33] | Wipatanawin, A., et al., Determination of the Effects of Adding Milk and Sugar on the Antioxidant Capacity of Oolong Tea by Chemical and Cell Culture-Based Antioxidant Assays. Chiang Mai J. Sci. , 2015. 42(3): p. 699-711. |
| [34] | Chiang, s.-h. and c.-y. Chang, Antioxidant Properties of Caseins and Whey Proteins from Colostrums. Journal of Food and Drug Analysis, 2005. 13: p. 57-63. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML