-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2017; 7(3): 69-74
doi:10.5923/j.fph.20170703.03

Fractionation of Annatto Extracts with Carbon Dioxide Using a Home-Made Equipment
Júlio C. F. Johner, Ádina L. Santana, M. Angela A. Meireles
LASEFI, School of Food Engineering, UNICAMP (University of Campinas), R. Monteiro Lobato, Campinas-SP, Brazil
Correspondence to: Ádina L. Santana, M. Angela A. Meireles, LASEFI, School of Food Engineering, UNICAMP (University of Campinas), R. Monteiro Lobato, Campinas-SP, Brazil.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Extracts obtained from whole annatto seeds were fractionated with supercritical carbon dioxide using a home-made experimental apparatus. The crude extracts, obtained in a 100 mL extractor at 313 K and 20 MPa were fractionated in two separator vessels, i.e., S-1 operating at 312 K and 9 MPa, and S-2 operating at 282 K and 4 MPa. Chemical composition of the resulted products was evaluated in terms of a qualitative approach using thin-layer chromatography. High-pressure phase behavior experiments were applied to the fractionated extracts using a synthetic-visual apparatus. Results indicate that annatto extracts were successfully fractionated in terms of visual aspects and differences in chemical profile. Chemical composition suggest that annatto extracts present relevant profile of terpenes and phenolics. The extracts presented similar phase behavior with the occurrence of vapor–liquid-equilibria, and vapor–liquid–liquid phase behavior for the studied compositions.
Keywords: Supercritical fractionation, Annatto, High-pressure phase behavior, Phenolic compounds
Cite this paper: Júlio C. F. Johner, Ádina L. Santana, M. Angela A. Meireles, Fractionation of Annatto Extracts with Carbon Dioxide Using a Home-Made Equipment, Food and Public Health, Vol. 7 No. 3, 2017, pp. 69-74. doi: 10.5923/j.fph.20170703.03.
Article Outline
1. Introduction
- Annatto (Bixa orellana L.) plays important economic and cultural roles as a natural additive colorant in food industry. Brazil is one of the largest producers and exporters of bixin, the pigment extracted from whole seeds [14]. Considering the necessity of natural substances for the industries, there is a recent demand for nutraceuticals and health-promoting ingredients from annatto seeds related to the quality of extracts. Supercritical fluid extraction (SFE) is an option to obtain high-value products with health-promoting properties [5]. Most of recent studies on annatto are focused on improving environmentally friendly processes for bixin extraction, for instance: microwave-assisted extraction [21], supercritical fluid extraction [1], pressurized liquid extraction [3], and ultrasound-assisted extraction [26]. Furthermore there are reports focusing on the potential application of annatto as a healthy alternative to synthetic dyes in formulations of bread [20], chicken [4] and cheddar cheese [22].Some kinds of compounds from extracts obtained via SFE may present high cost and often are present in low concentration, which reduces its value for marketing as crude extract [11]. The separation of these extracts for obtaining of new products with distinct compositions is a way to improve the quality of extracts in terms of the high concentration of the compounds of interest [25, 7]. Fractionation of natural extracts with supercritical fluids using the combination of separators results in the acquisition of high-quality products with increased purity and differentiation of bioactive compounds [23].One of the difficulties in the extract fractionation is related to the solubility, i.e., the ability to separate volatile oils from other compounds that CO2 can dissolve [12]. The main factors that interfere in the separation process are pressure and temperature. At low temperatures (-5 up to +5°C) waxes are insoluble in CO2 [13].The knowledge of the phase equilibrium behavior of the volatile and nonvolatile fractions of foods is of crucial importance for many applications in the food industry [15]. In the case of annatto extracts it can provide theoretical data that can help the selection of process conditions. However, there are very few investigations reporting the bioactive profile of annatto extracts obtained from supercritical fluid extraction [2] and their high-pressure phase behavior [16, 17].In this context, the goal of this work was to propose a new process for the fractionation of annatto crude extract using supercritical carbon dioxide as solvent. The phase behavior of the fractionated extracts was studied using a high-pressure equilibrium cell at three levels of temperature. In addition, the chemical profile of these products was determined using thin-layer chromatography (TLC).
2. Material and Methods
2.1. Raw Material
- Annatto variety Piave was obtained from IAC (Agronomic Institute of Campinas), Department of Agriculture and Supply from the State of São Paulo, Brazil.
2.2. Supercritical Fluid Extraction
- Extraction of whole annatto seeds and subsequent fractionation of crude extracts were carried out in the home-made SFE-0.1L unit (Figure 1), built and validated elsewhere [9]. This unit is composed by two parts: the first is constituted of a 100 mL extractor and the second part is composed of two 90 mL separators (S-1 and S-2). Whole annatto seeds were loaded into the extractor and the extracts were collected in the two separators. Carbon dioxide, CO2 (99% purity, White Martins, Campinas, Brazil) was used as solvent and SFE assays were performed at 313 K and 20 MPa at the solvent/feed ratio (or S/F) of 21. These conditions were selected because of high extract yields obtained previously [1, 2].
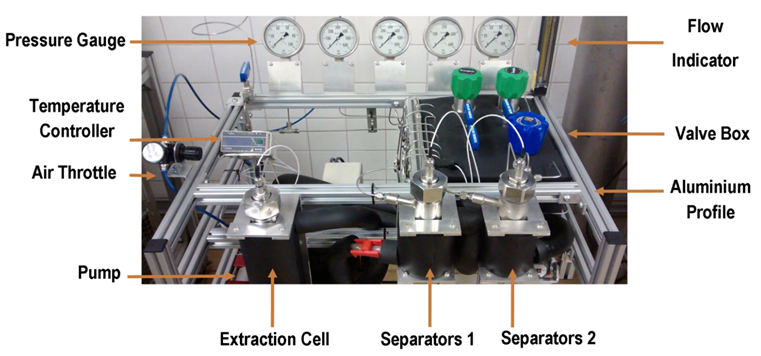 | Figure 1. Home-made SFE-0.1L unit |
2.3. Thin-layer Chromatography
- Silica gel plates with aluminum backs (Alugram®, Xtra SIL G, Macherey-Nagel, Germany) were used as stationary phase. The mobile phase was composed of chloroform (Merck, Darmstadt, Germany), ethanol (Chemco, Hortolandia, Brazil) and glacial acetic acid (Synth, Diadema, Brazil), in the proportion of 95/05/01, v/v/v [24].To facilitate the identification of the compounds the following standards were used: ar-turmerone, trans-caryophyllene, camphor, carvacrol, α –humullene, limonene, r-carvon, terpineol (mixed isomers, principally α, approximately 95%), p-cymene (99%) and α-bisabolol (>85%), obtained from Sigma-Aldrich (Darmstadt, Germany). Bixin standard was obtained with exhaustive extraction with acetone, according to the procedure described elsewhere [9]. The bands of compounds generated by the constituents that could not be detected in the visible region were visualized using a UV (Multiband UV – 254-366 nm, UVGL-58, Mineralight® Lamp, Upland, CA, EUA) equipped with a cabinet (UVP-Chromato-VUE, CC-10, Upland, CA, EUA) for short wavelength (254 nm) and long wavelength (366 nm) visualization. The phenolics and volatiles were detected using the sulfuric p-anisaldehyde spray reagent, according to the formulation proposed by Hamilton & Hamilton [8] and the sulfuric vanillin spray reagent, according to the formulation proposed by Krishnaswamy [10]. The p-anisaldehyde and vanillin used in this work were purchased from Sigma-Aldrich (Darmstadt, Germany) and Synth (Diadema, Brazil), respectively. The sulfuric acid used in this work was obtained from Exodo (Hortolandia, Brazil). Approximately 10 μL of the samples were spotted on the thin-layer chromatography (TLC) plates with the aid of chromatographic syringe (Hamilton, Darmstadt, Germany) with approximately 1 cm distance from each band. Afterwards the plates were developed into glass chambers by elution in mobile phase. After elution, the compounds of interest were detected by spraying the plates using the sulfuric p-anisaldehyde, or the sulfuric vanillin, reagents. After detection with p-anisaldehyde the plates were subjected to thermal activation using an oven (Tecnal, TE-385-1, Piracicaba, São Paulo) at 373 K for 5 minutes.
2.4. High-Pressure Phase Equilibrium
- High-pressure phase behaviour experiments of annatto fractionated extracts in the presence of compressed CO2 were performed adopting the synthetic method with phase transition visualization using a high-pressure variable-volume cell, from which apparatus was built [15] and validated [16].Three levels of temperature (303, 313 and 323 K) were used to perform high-pressure phase equilibrium assays with a minimum of three replicates. Due to the poor availability of sample, the extracts were loaded into the cell with the aid of a 10 μL chromatographic syringe (Hamilton, Sigma-Aldrich, Darmstadt, Germany). The overall composition of CO2 expressed in terms of mass fraction (ω1) varied from 0.92 to 0.99.
3. Results and Discussion
3.1. Extraction and Fractionation
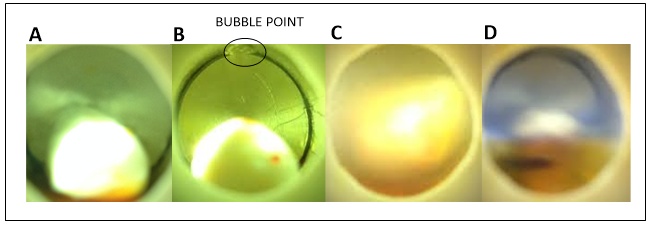
- Crude annatto extract obtained at S/F=21 yielded 2% from whole seeds. This yield was slightly lower than 3.8% obtained previously at S/F=37 [9] because high values of S/F imply exhaustion of the raw material, and consequently, high extract yields. Besides this, lower yields may be associated with loss of extract inside the S-1 Figure 2C) and S-2 (Figure 2D) separators because the extracts are collected directly with the aid of the micrometering valve, which reduces the course of the extract until the flask collector.The fractionated extract collected from the S-1 separator (Figures 2A and 2C) corresponds to 58% from crude annatto extract, while the extract collected from S-2 separator (Figures 2B and 2D) corresponds to 41% from the crude annatto extract.Depressurization prevented loss of extract in the pipeline located after the second separator. Cooling of the second separator is another factor that allowed complete separation of extract from solvent, because of differences in densities between solvent and extracts.Obtaining of CO2 with low residual content of extract is a determining factor for the recycling operation of this solvent, since the separation procedure for complete CO2 removal may carry a part of the extract to the recycling line, which causes loss of extract and further damages the recycling equipment.
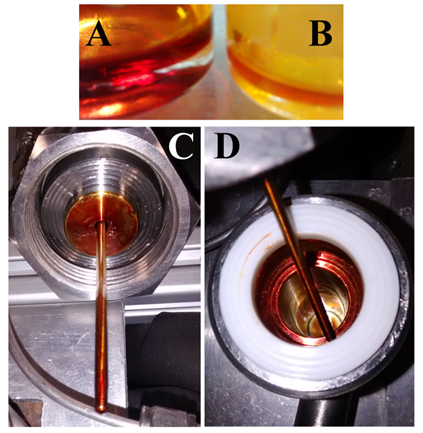 | Figure 2. Annatto fractionated extracts obtained from S-1 (A) and S-2 (B, C and D) separators |
3.2. Thin-Layer Chromatography
- The fingerprints of fractionated annatto extracts differ from those reported elsewhere for crude annatto extracts obtained with supercritical CO2 and developed with n-hexane/ethyl acetate/formic acid (v/v/v) mobile phase [2, 9].Both p-anisaldehyde and sulfuric vanillin spray reagent are universal reagents for natural products, making color differentiation possible, i.e., they are reagents used to detect antioxidants, steroids, prostaglandins, carbohydrates, phenols, glycosides, sapogenins, volatile oil components (or terpenes), antibiotics and mycotoxins, depending on the preparation of sample, type of stationary phase and mobile phase formulations [24, 6].The manner of detection of sulfuric p-anisaldehyde spray reagent differs from sulfuric vanillin by the need of thermal activation after spraying. After thermal activation with this spray reagent, only annatto fractionated extracts showed compounds that could be visualized in UV at 240 nm and 360 nm (Figure 3A). The formulation of vanillin-sulfuric acid spray reagent used in this work it is a highly destructive reagent, which is applied along with thermal activation, resulting in partial destruction of TLC plate, as can be observed in Figure 3B.Sulfuric acid is an oxidant and its reaction with terpenes in the presence of vanillin results in the formation of purple spots, which are observed in the case of trans-caryophyllene, α-humullene, terpineol and α-bisabolol standards (Figure 3B).After reaction between vanillin with sulfuric acid and p–anisaldehyde coupled with thermal activation, the resultant yellow or red coloration bands are associated with phenolics, and dark-blue or purple bands are associated to volatiles, very similar to the bands of waste turmeric products detected with vanillin-sulfuric acid spray reagent [19, 18].After spraying with the sulfuric vanillin reagent, the purple bands appeared on the extracts (Figure 3B), similarly as trans-caryophyllene, α-humullene, terpineol and α-bisabolol standards (Figure 3B) indicating the presence of volatile terpenes. The presence of pink and light-red bands are associated to phenolics (Figure 3B).Thermal activation of fractionated extracts in the TLC plates sprayed with p-anisaldehyde reagent resulted in the slight modification of yellow coloration which turned into dark-blue (Figure 3A), similar to that reported previously for crude annatto extracts obtained with supercritical CO2 [2]. Bands of fractionated annatto extracts indicate the presence of α-bisabolol, because of similarities in fingerprint and band coloration (Figures 3A and 3B).
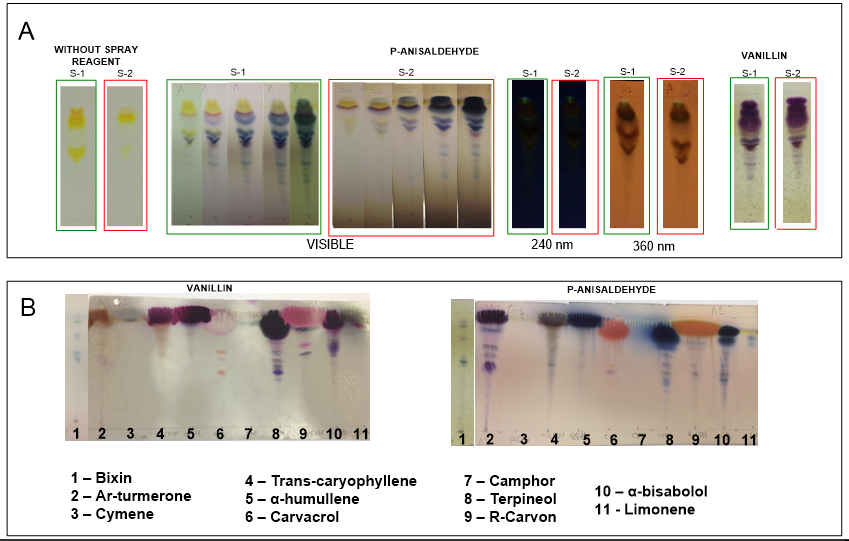 | Figure 3. Thin-layer chromatography plates of annatto extracts collected from the S-1 and S-2 separators (A), bixin and volatiles standards |
3.3. High-Pressure Phase Equilibrium
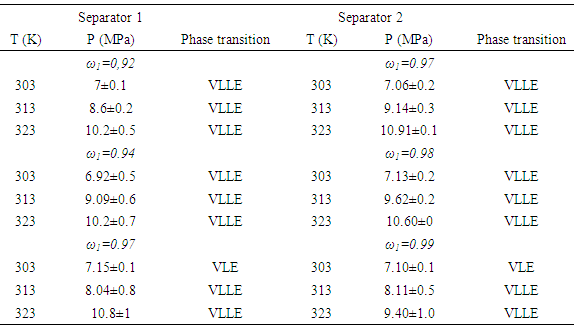
- The experimental measurements for the multicomponent system containing annatto extracts showed a slight variation of phase transitions at higher proportions of CO2. Vapor-liquid equilibria (VLE) and vapor liquid-liquid equilibria (VLLE) phase transitions were observed, according to Table 1.
|
4. Conclusions
- In this work, crude annatto extracts were fractionated with supercritical and compressed carbon dioxide using a home-made experimental apparatus.Fractionation of annatto extracts resulted in two distinct products, with relevant chemical composition in terms of terpenes and phenolic constituents, which are potential health-promoting agents, that can be included in foods drugs and cosmetic products. High-pressure phase equilibrium data for the pseudobinary systems involving supercritical carbon dioxide and annatto extracts were similar and presented complex behavior with vapor-liquid and vapor liquid-liquid phase transitions because of multicomponent nature of the extracts.
ACKNOWLEDGEMENTS
- The authors acknowledge CAPES (2952/2011), CNPq (302423/2015-0), CNPq (140287/2013-2) and FAPESP (RT2015/13299-0) for financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML