-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2017; 7(3): 51-58
doi:10.5923/j.fph.20170703.01

Effectiveness of Ozone Gas Application Methods against Combined Multi-Contaminants in Food
Christ D.1, 2, Savi G. D.1, Scussel V. M.1
1Laboratory of Mycotoxicology and Food Contaminants, Food Science and Technology Department, Center of Agricultural Sciences, Federal University of Santa Catarina, Florianopolis, SC, Brazil
2Storage Laboratory and Drying Prototypes Facilities, Technological and Exact Sciences Center, Western State University, Cascavel, PR, Brazil
Correspondence to: Scussel V. M., Laboratory of Mycotoxicology and Food Contaminants, Food Science and Technology Department, Center of Agricultural Sciences, Federal University of Santa Catarina, Florianopolis, SC, Brazil.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Despite the ozone (known as GRAS - generally recognized as safe) gas treatments published for each food living organism or chemical decontamination, they are not necessarily present or contaminate food individually. Most of the time, they are present all together. Therefore, the most important point, regarding an O3 application method to be chosen is its effectiveness to all contaminants that may be in the target food. Therefore, ozone gas decontamination methods that gather combined multi-contaminants and their effectiveness in food were surveyed. Both, for inactivation of living organisms (insects, mites, fungi, yeasts and bacteria) and degradation of toxic compounds (pesticides and mycotoxins). They are the main need for an effective and comprehensive commercial decontamination application. This review brings details on O3 formation energy sources, its application conditions (concentration / time of exposure), container characteristics (volume / material type / sealed or hermetic) and effectiveness for different food (type / contamination level / batch size/moisture content). From the studies and data reported, ozone has shown to be an efficient green agent for combined multi-decontamination, thus prolonging the food storage and shelf life. It does not cause/produce adverse effects on food quality.
Keywords: Ozone, Decontamination, Fungi, Mycotoxins, Methodology
Cite this paper: Christ D., Savi G. D., Scussel V. M., Effectiveness of Ozone Gas Application Methods against Combined Multi-Contaminants in Food, Food and Public Health, Vol. 7 No. 3, 2017, pp. 51-58. doi: 10.5923/j.fph.20170703.01.
Article Outline
1. Introduction
- The knowledge on ozone (O3) gas methods against food multi-contaminants combined, such as living organisms (insects, mites, fungi, yeast, bacteria) and toxic compounds (pesticides, mycotoxins, toxic wastes) is necessary to reach a safe, effective and comprehensive commercial application. O3 has been reported showing its safe decontamination efficiency for raw and processed food, either of high (fruits / fresh vegetables) and low (grain / nuts / pulses) humidity, without leaving residue [1-9].
1.1. Ozone Gas
- The first information regarding O3 was carried out by the Dutch, van Marum in 1783. The author observed that the air, surrounding an electrostatic machine (during a series of electric sparks application), acquired a sharp and characteristic odor [10]. Schönbein in 1840, reported at the Munich Academy that during the parallel O2 evolution reaction, occurs a second unknown gas production with a pungent smell which was then called ozone (from Greek: ozein meaning smell). In addition, showed that the gas can be formed in certain autoxidation processes (by phosphorus in air presence reaction). However, he also was not able to establish the gas nature or composition [10]. Only, around 16 years later, O3 was then identified as a triatomic allotrope form of O2 by Thomas Andrews, with Peter G. Tait collaboration in 1856 [10]. Their work on gas and liquid phase transitions was able to show that O3 was formed by oxygen atoms. Next, Soret in 1863 established the relationship between O2 and O3 by considering that 3 volumes of O2 produce 2 volumes of O3 [11].When O2 is broken into individual oxygen atoms, which combine with other O2 , the O3 is formed [12]. Its molecule undergoes a spontaneous dissociation (high O3 instability) process, again resulting in O2 formation [13, 14]. The O3 bluish gas half-life time varies from a few seconds to hours, and its stability depends on different factors, among them, temperature. An increase of 10°C results in a reduction of at least 43% in the O3 half-life [15]. At atmospheric conditions, its half-life is about 30 min and this reaction proceeds more rapidly at higher temperature and lower pressure [16-19]. Therefore, being an unstable gas, it requires to be produced at its application site, thus reducing costs and risks related to transport and storage [9, 20, 21]. As a very reactive oxidizing agent, O3 has proved effective against a broad spectrum of living organisms and chemicals. It kills bacteria, fungi, yeasts, viruses and protozoa [17, 18, 22-28] storage pests, such as insects & mites. It also has the potential to degrade mycotoxins, pesticides and toxic chemical wastes [2, 7-9, 26, 29-34].It has been considered by different international organizations and countries regulations as GRAS (generally recognized as safe) and can be utilized in direct contact to drinking water and food (the US Food and Drug Administration – FDA & Agriculture Department - USDA, Food Agriculture Organization – FAO and World Health Organization - WHO) [9]. Apart from those international institutions and countries (Europe, China; Japan, Australia, Brazil) ministries of agriculture and health, recognizing the use of O3 for food, there is a wide supporting literature attesting the benefits of ozonation as an efficient method/procedure for food (raw & processed) living organisms inactivation and chemicals degradation [9].
1.2. Ozone Production Energy Sources
- The gas can be produced either, naturally and freely in the stratosphere through the interaction of solar ultraviolet radiation with the molecular O2 [35], or artificially through electric dischargers reaction or ionizing radiation, being the corona discharger apparatus, the most known and utilized in different food processes [9]. The gas production, when performed by the electric discharge process (corona discharger), occurs through the tunnel gap between 2 electrodes subjected to a high potential difference of approximately 1000 V (electrode A: high voltage; electrode B: ground) (Figure 1). The O3 then, is generated by the passage of air or pure O2 between the electrodes. Thus, when electrons have enough energy to dissociate the O2 molecules, collisions among them begin to occur, causing the dissociation of O2 with subsequent O3 formation [9, 26, 36]. There are available different types, sizes and potential intensities O3 generators (ozonisers); from small sizes/low capacity to large sizes/high capacity. Some of them can be portable, installed along the industries processing plants or mobile (that can be hired by different grain storage unities) [9, 37].
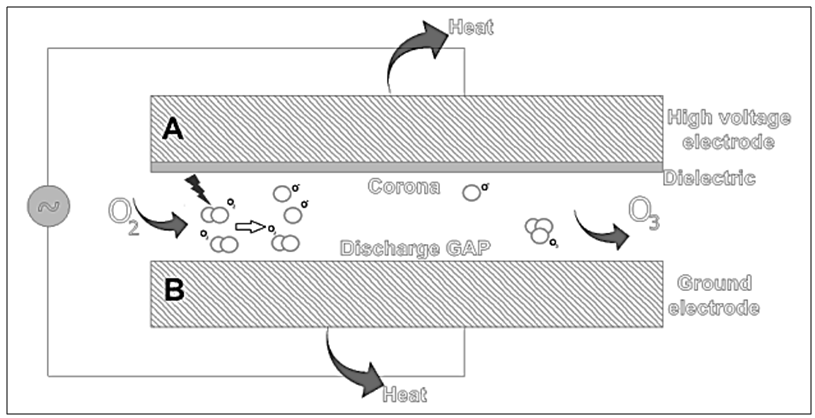 | Figure 1. Ozone (O3) generation: corona discharge |
2. Ozone Multi-contaminants Inactivation and/or Degradation in Raw and Processed Food
- Despite the O3 treatment findings and publications for each food living organisms or chemical contaminants published, they are not necessarily present or contaminate food individually. Most of the time, they are present all together, such as combinations of fungi / mycotoxins & pesticides; or insects / mites / bacteria & insecticides. Therefore, the most important point, regarding an O3 application method to be chosen (right concentration / exposure time / whether gas or aqueous O3 / efficiency), is its effectiveness (inactivation and/or degradation) to all contaminants that may be in the target food. Indeed, there has been quite a broad list of publications on O3 gas application in food, most of them reports its influence on individual contaminants though [9]. Fortunately, there are studies reporting the O3 effectiveness to multiple contaminants present in same food at different combinations such as bacteria, yeast & fungi; fungi & insects; fungi & mycotoxins; fungi, mycotoxins & insects and fungi & pesticides [2, 7-9, 15, 25, 38-46]. The gas concentrations; exposure time versus food contamination levels and the groups (cereals / pulses / nuts / fruits / spices) have been also detailed including the percentage of reduction. Some of them do inform their effectiveness related to the initial (bacteria / yeast /fungi load and toxin level - whether high or just above the maximum limit allowed) and final (after gas treatment) food contamination, which are quite important information, to achieve the O3 method concentration effectiveness [9].We report here studies on O3 gas application against multi-contaminant combinations which can be more effectively utilized on the food area against both groups: living organisms and toxic compounds. Table 1 shows the treatments against the multi-contaminants (type / concentration / time of exposure / inhibition rate/percentage) reported in the food area. Note: due to the variations on unities utilized by different authors, they (unities) were kept as reported.
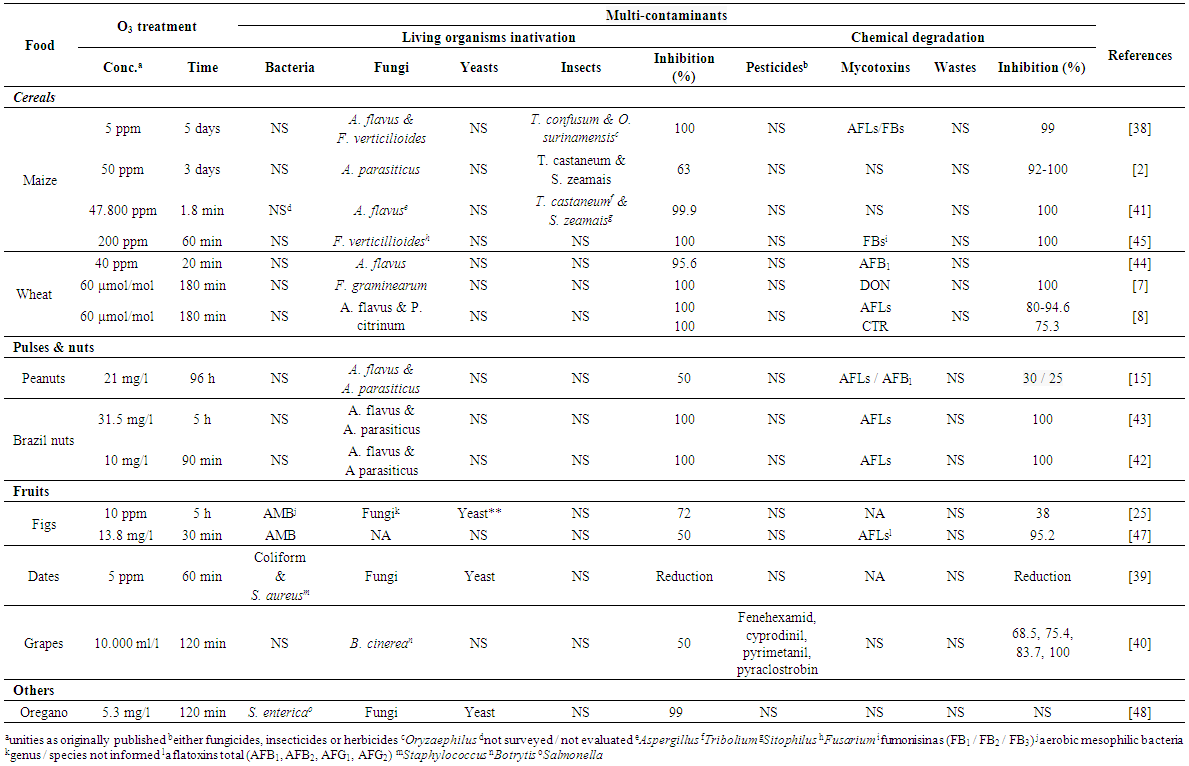 | Table 1. Ozone applications for Multi-Contaminants degradation in different foods reported in the literature |
2.1. Living Organisms
- Studies on ozonation against combinations of multiple living organisms (bacteria, yeast, fungi and/or insects) are reported mainly on fruits, cereals and spices.Bacteria, yeast and fungi: Öztekin et al. [25] evaluated O3 application in figs at rate of 10 ppm during 5 h and observed reduction of 72% of aerobic mesophilic bacteria (AMB), fungi and yeast. Also, Zorlugenç et al. [47] applied O3 in figs at rate of 13.8 mg/l during 30 min, observing reduction of 50% in AMB and 95.2% of AFLs. Najafi and Khodaparast [39] applied the gas at rate 5 ppm during 60 min in dates and reported reduction in bacteria (coliforms, Staphylococcus aureus), fungi and yeasts. In studies conducted to find the effect of O3 in spices (oregano), Torlak et al. [48] reported reduction of 99% of bacteria (Salmonella enteric), yeast and fungi when applied 5.3 mg/l-1 during 120 min. All authors, although report details of bacteria genera, do not specify fungi and yeasts. That is due to the fact that the aim was to inactivate all of them, despite their denomination details (Table 1).Fungi and insects: in a study that evaluated the O3 fumigant efficacy (disinfestation - insects / and disinfection -fungi) in stored maize (8.9 tonnes - 350 bu) at 50 ppm for 3 day, resulted in 92 to 100% mortality. That included the adults red flour beetle (Tribolium castaneum Herbst), maize weevil (Sitophilus zeamais Motsch.) and the Indian meal moth (Plodia interpunctella Hubner) at larval stage. The treatment also reduced by 63% the contamination of fungi (Aspergillus parasiticus Speare) on the kernel surface [2]. On the other hand, McDonough et al. [41] studied the concentration of O3 in the screw conveyor (47,800 ppm) application in a short time (time average: 1.8 min). Under these conditions, 100% mortality of T. castaneum Herbst & S. zeamais Motsch and 96% reduction of A. flavus spores were observed in a single pass through the screw conveyor.
2.2. Toxic Compounds Combined
- Despite of the studies related to multiple living organisms decontamination methods by O3, only a few have been reported on combination of multiple toxic compounds including pesticides and wastes in food. However, food pesticide multi-degradation from their different Classes (organochlorines / organophosphorus / dithio- / carbamates / pyrethroids) and Groups (fungicides / insecticides / herbicides / among others) have been reported being degraded by O3. They are one of the main concerns regarding food safety of vegetable origin due to their commercial application during crops development in the field and will be mentioned here as multi-pesticides from different Classes: Fungicides - grapes fenehexamid, cyprodinil, pyrimetaniland pyraclostrobin, dithiocarbamate treated, had their residues degraded by O3 at 10 ppm exposed during 60 min. The reduction was 68.5, 75.4 and 83.7% for the three compounds, respectively [40]. Insecticides - when seeds (soft wheat, maize, dry wheat) were fenvalerate, methomyl, deltamethrin and fenitrothion contaminated and O3 treated (10-180 min, at 5-125 mg/l and 20-100 l/h flow) had quite effective reduction (up to 99%) [49-52]. Insecticides O3 gas treated could be effectively reduced and degraded into non toxic by-products, especially for the rat liver functional interlinks [53, 54]. Herbicides - when bromoxinile and trifluralin were O3 treated at doses ranging from 80 to 480 μM (10 min), the gas was able to significantly react with bromoxynil, achieving degradation of 98% (after 2 min), however trifluralin was less O3 reactive, reaching only half (50%) of its degradation after 5 min [55]. Toxic wastes - on the other hand, wastes that may be present in factories water effluents such as from wine distilleries (also pharmaceutical and textile industries), the O3 gas lead to living organisms inactivation and toxic compounds degradation thus thus can be a quite beneficial to nature and the humans safety in the area [20, 31, 34, 56-59].
2.3. Living Organisms & Toxic Compounds
- Regarding combination of multiple living organisms and toxic compounds decontamination methods reported in the literature, they were in the following combinations.Fungi and mycotoxins: concentrations of O3 gas to be applied for both fungi & toxins vary quite widely among the authors, such as low as 5 ppm, for a long exposure period such as 5 days, which can inhibit 100% of fungi (Aspergillus flavus and Fusarium verticillioides) and AFLs in maize, carried out by Mason et al. [38]. Mylona et al. [45] also studied O3 application in maize, at 200 ppm during 60 min, obtained 100% of A. parasiticus inativation and FB1/FB2/FB3 degradation. As far as wheat is concerned, El-Desouky et al. [44] observed a reduction of 95.6% in the A. flavus infection and 100% degradation of AFB1. In another study with wheat, F. graminearum growth was totally inhibited after 180 min of O3 exposition at 60 µmol/mol and so DON contamination. In addition, the same authors, showed that the A. flavus growth was totally inhibited under those conditions [7]. Same research group reported that AFLs levels significantly decreased after 180 min at 60 µmoL/moL (94.6, 84.5, 80.0 and 81.0% of reduction for AFB1, AFB2, AFG1 and AFG2, respectively) and so A. flavus growth. The total inhibition of P. citrinum growth occurred also after O3 treatment longest time (180 min of exposure). In this same study, the CTR levels reduced after O3 treatment in both concentrations (40 and 60 µmoL/moL) after 180 min of exposure [8]. Regarding nuts, the application of O3 in Brazil nut in studies of Scussel et al. [42] and Giordano et al. [43] at rate of 10 and 31.5 mg/l during 1.5 and 5 h obtained 100% of A. flavus, A. parasiticus and AFLs decontamination. Fungi and fungicides: Gabler et al. [40] applied O3 in grapes during 2 h at rate of 10.000 ml/l, aiming to remove Botrytis cinerea and fungicides (fenehexamid, cyprodinil, pyrimetanil and pyraclostrobin). Authors reported that the treatment reduced in 50% the fungi and up to 100% the fungicides. Insect, fungi and mycotoxins: Mason et al. [38] applied 5 ppm during 5 days through maize grains aiming to destroy fungi (A. flavus and F. verticilioides), insect (T. confusum and Oryzaephilus surinamensis) and mycotoxins. Authors observed 100% elimination of fungi and insect and 99% of AFLs degradation.Regarding O3 gaseous effects on food composition, seed germination and/or its safety, several studies have reported its Green performance [9].
3. Ozone Gas Parameters for Food Decontamination Efficiency
- Among the food types that have been efficiently O3 treated and being reported in the literature are those of vegetables and animal origin (either, raw, dry or processed). The application parameters (concentration and exposure time) vary according to the food protection (decontamination) purposes, i.e., whether for living organisms inactivation and/or toxic compounds degradation.Due to the literature O3 diversity on decontamination parameters, several points need to be considered and paid attention prior the choice of adequate and efficient method of O3 application in food as follows. (a) Doses and contamination levels: the gas application methodology can be efficient, in (a.1) a single or may need multiple O3 doses; including (a.2) adjustments on its concentration according to the extent/intensity (load/level) of living organisms / toxic compounds to be destroyed, and/or the food group to be treated [42, 43, 60-63]. (b) Time: however, the efficacy of O3 application depends also on other factors, such as the (b.1) time of exposure; (b.2) humidity and (b.3) temperature during the procedure [7, 8, 41]. Food characteristics x chamber size - in addition, the food (b.4) characteristics (whether with or without - husk/pericarp/testae/shell; in small or large particles - ground/whole) and the (b.5) container capacity and size, play important roles on the efficiency of the treatment gas conditions to be chosen and applied, which should be adjusted for adequacy. (c) Environment: another point to be considered for O3 best application is whether the food is present in bulk (loose) or separated in bags (small / large packages). Static or in motion - whether the gas is applied in static (inside ship containers or silos) food amount/quantity or in movement (conveyor transport belt / loading screw or during food storage load/unloading onto ship, train, container, truck). (d) State of food matrix: for raw (dry cereal / pulses / nuts / fruits - low humidity) or fresh (minimally processed vegetables sliced / chopped / peeled - high humidity) food, the application need attention on gas concentration and time adjustments [7, 8, 38, 41, 42, 47, 60, 61, 64-66].
4. Ozone Gas Parameters for Food Decontamination Efficiency
- When deciding to apply an O3 gas decontamination method, one needs to know on how the gas concentration (to be achieved), will be measured and confirmed in the chamber and micro-environment surrounding the food. Despite of several methods (physical, physicochemical and chemical methods) that are reported for O3 measurement, there are differences and efficiencies to be considered among them:
4.1. Gas Concentration Measuring
- The (a) Physical methods, (a.1) measure its absorption (directly in the UV, visible or infrared spectrum region) and the (a.2) quantify the products released (when O3 reacts with a chemical reagent such as potassium iodide for example). In the (b) Iodometric method, that has been approved by the International O3 Association [67], O3 oxidizes the iodide ions releasing iodine; that is then titrated with sodium thiosulfate to a starch endpoint. This method measures, apart from O3, also other formed oxidizing species (from the O3 decomposition solution - ŸO3-, HO2Ÿ, and ŸO2-), hence, the measurement of residual O3 cannot be accurately done [13, 14]. However, the most commonly used is the (c) Indigo method, which is precise, fast and sensitive (low detection: 0.005 µg/ml) [68]. As the indigo solution reacts additively with the C=C bound (of the sulfonated indigo dye) causing its discoloration, that color changing is measured spectrophotometrically. It is not compromised, neither by the hydrogen peroxide, organic peroxide, mangans ions and oxidase species presence in the sample. Compared to the Iodometric, the Indigo Method is more suitable for measuring O3 residual [9, 13, 14].
4.2. Measuring Instruments
- Several manufactures produce instruments that measure O3 by determining the amount of UV light absorbed. Gaseous O3 absorbs short-UV wavelengths (maximum absorption at 253.7 nm) with a gas-phase absorption coefficient of 3000+30 m-1 cm-1 (at 273 °K and 1 atm) [67]. On the other hand, the Calorimetric Methods depend upon the O3 decomposition in presence of a catalyst producing heat. Instruments using amperometric methods to measure the oxidation-reduction potential of O3 are available commercially [13, 14].
5. Conclusions
- From the studies and data reported, O3 has shown its efficacy as a Green agent against combined multi-contaminants (present in the same application method).It is possible to obtain safe food through O3 gas decontamination, as long as the adequate conditions are observed and considered (regarding the food matrix, its surrounding environment, the contamination level and humidity present) in the application to be utilized and/or developed.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML