-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2017; 7(2): 40-50
doi:10.5923/j.fph.20170702.03

Risk Prevention of Fungal Contamination of Raw Cocoa Beans in Côte d’Ivoire: Case of Polyhexamethylene Guanidine Hydrochloride (PHMGH)
Konan M. Yao1, Ollo Kambiré1, Kouassi C. Kouassi2, 3, Rose Koffi-Névry3, Tagro S. Guéhi3
1Biological Sciences Training and Research Unit, Peleforo Gon Coulibaly University, Korhogo, Côte d'Ivoire
2Agroforestry Training and Research Unit, Biochemistry-Microbiology Teaching Unit, Jean Lorougnon Guédé University, Daloa, Côte d'Ivoire
3Food Science and Technology Training and Research Unit, Nangui Abrogoua University, Abidjan, Côte d'Ivoire
Correspondence to: Konan M. Yao, Biological Sciences Training and Research Unit, Peleforo Gon Coulibaly University, Korhogo, Côte d'Ivoire.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Quality defects associated with Ivorian cocoa cause substantial economic losses for the state of Côte d’Ivoire. This study was conducted to help provide an acceptable quality cocoa on the international market. The disinfectant Polyhexamethylene Guanidine Hydrochloride (PHMGH) was used to prevent the risk of fungal contamination of raw cocoa beans. Three methods of fermentation and also three drying surfaces were used for this study. Real environment, PHMGH exerts fungicidal activity at 4 mg/mL and a fungistatic effect at 2 mg/mL. After 6 days of sun drying, the moisture content of the control samples (7.47 to 7.87%) were relatively low compared to those treated samples (7.60 to 8.10%) with PHMGH. However, no significant difference (p>0.05) was observed between the values of the moisture content of the different cocoa samples. For each method of fermentation and for each drying surface, the pH (4.60 to 5.00) and titratable acidity (11.20 to 12.40 meq NaOH/100 g) of cocoa beans do not vary significantly (p>0.05) in a concentration of PHMGH to another. This non-toxic disinfectant for humans, which has no effect on pH and titratable acidity of raw cocoa beans, may be recommended to prevent raw cocoa beans fungal contaminants at a concentration of 4 mg/mL.
Keywords: Raw cocoa beans, Quality, Mold, Conservation, PHMGH, Fungicidal activity, Côte d’Ivoire
Cite this paper: Konan M. Yao, Ollo Kambiré, Kouassi C. Kouassi, Rose Koffi-Névry, Tagro S. Guéhi, Risk Prevention of Fungal Contamination of Raw Cocoa Beans in Côte d’Ivoire: Case of Polyhexamethylene Guanidine Hydrochloride (PHMGH), Food and Public Health, Vol. 7 No. 2, 2017, pp. 40-50. doi: 10.5923/j.fph.20170702.03.
Article Outline
1. Introduction
- The cacao tree (Theobroma cacao L.) was introduced in Côte d’Ivoire in the late 19th century, in the eastern region of the country [1, 2]. After a difficult start, cocoa farming has grown very rapidly, especially after the 2nd World War [1]. Since 1970, Côte d’Ivoire ranks first in cocoa producing countries [3]. During the 2014/2015 season, 4.2 million tons of cocoa have been produced worldwide. 73% of world production came from West Africa, 17% from Central and South America, and 10% from Asia. The largest producing country was Côte d'Ivoire which produced 42% of world production [4]. The cocoa bean is the raw material for chocolate industry that manufacture or semi-finished products or finished products. The various semi-finished cocoa products (powder, butter and cocoa mass) are very important ingredients in many types of food, such as cakes, biscuits, baby food, ice cream and candy [5-7]. Directly consumable finished products include chocolate in tablet or powder, confectionery, chocolate delicacies etc. Although cocoa is largely produced in developing countries, derivatives are mainly consumed in industrialized countries. According to statistics from the International Cocoa Organization (ICCO), the United States consumed more than 30% of world cocoa production and occupy the forefront of consumers [4]. Cocoa not only occupies an important place in the Ivorian economy, but also the main source of income for producers. It represents the macroeconomic level, from 30 to 33% of export earnings and contributes 10% of Gross Domestic Product (GDP) [1]. Increasingly, efforts are made by producers in the use of chemicals such as fertilizers, herbicides and insecticides to substantially increase production. However, cocoa and cocoa pod are facing serious microbial diseases such as viral disease called "swollen shoot" or disease swelling of cocoa tree branches and the fungal disease "brown rot of the cocoa pod" caused by Phytophthora spp. To control these diseases, fungicides (Nordox 75 WG, Gold Plus 65 WP, ...) are used in the case of "black pod of the cocoa pod" [8]. "Swollen shoot" control is essentially preventive and is still agronomic [8]. Despite these actions, the producers are still facing a serious problem of impaired quality of raw cocoa beans by mold. This creates huge losses of crops and therefore promotes substantial economic losses.Failure predominant quality associated with the Ivorian cocoa is the significant percentage of slaty beans and moldy beans, due to poor fermentation and poor drying [3, 9]. The requirement of the international market for quality food led Côte d’Ivoire, the first world producer of cocoa, increase quality control of field edge cocoa beans. This represents a challenge for the country.The quality of cocoa beans takes into account their degree of fermentation. Indeed, fermentation is one of the important processes to improve the quality of the cocoa beans [10, 11]. This step could determine the acceptability or rejection of beans on the international market. According to Pontillon [12], poorly fermented cocoa beans lead to poor quality of the chocolate. Fungal contamination of cocoa is also a serious quality defect. According to Mossu [13], cocoa presenting moldy beans rates above 4% and slaty beans above 8% is unsuitable for industrial processing and is therefore subject to rejection or downgrading in the international market. Thus, according to the Directorate General of the Treasury and the Ivorian Accounting, because many associated quality defects repeatedly cocoa from Côte d’Ivoire, the Ivorian state loses annually between 50 and 60 billion FCFA [14]. Also, several studies [15, 16] have they shown that massive contamination of cocoa beans by molds may lead to a considerable increase in free fatty acids (FFA) beyond 1.75%. This increase in the content of free fatty acids decreases the viscosity of the cocoa butter [12]; this is a serious quality defect and leads to rejection of the batch of concerned cocoa on the international market. Furthermore, other authors [6, 17, 18], reported that fungal contaminants in cocoa can lead to undesirable odors and production of carcinogenic mycotoxins such as aflatoxin B1 and Ochratoxin A (OTA). All these quality defects resulting in general, of misbehavior post harvest technology operations such as fermentation, drying and storage [19-22]. Given these various levels of contamination of cocoa, it should look for ways that can help control fungal contaminants and reduce post harvest losses. The use of means of technical, biological and chemical could solve this problem. The technical solution is used generally, is to control post harvest cocoa technology. However, this solution is limited by the large number and geographic dispersion of Ivorian cocoa farmers. The biological solution is expensive and require huge technical resources for the production of microorganisms capable of hampering the development of undesirable molds. The easiest way to implement now seems to be that the use of fungicides which are of course non-toxic to humans.In this perspective, Polyhexamethylene Guanidine Hydrochloride (PHMGH), chemical already used as a disinfectant, is timely. The many physical and chemical characteristics of this product: odorless, colorless, non-corrosive and non-toxic for humans with a neutral pH [23] make an innovative product in the disinfection and preservation of food products. The antimicrobial activity of this product was demonstrated by Oulé et al. [24] on many bacteria such as Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella choleraesuis, Methicillin-resistant Staphylococcus aureus (MRSA) and Escherichia coli. Moreover, Yao et al. [25] noted in their in vitro experiments that PHMGH has a fungicidal activity on fungal species such Absidia corymbifera, Rhizopus oryzae, Aspergillus tubingensis, Aspergillus tamarii, Aspergillus flavus and Penicillium chrysogenum, isolated from raw cocoa beans. Also, Koffi-Nevry et al. [26] reported the antifungal efficacy of the PHMGH on fungi such as Mucor sp., Botrytis sp., Penicillium sp., Geotrichum sp., Aspergillus sp. and Colletotrichum sp. isolated from papaya.The main aim of this study was to help prevent contamination of raw cocoa beans by toxigenic molds in order to reduce post harvest losses, using Polyhexamethylene Guanidine Hydrochloride.
2. Material and Methods
2.1. Material
- Cocoa pods samples were prepared in April 2013 in a peasant plantation in Soubré (the main cocoa producing region located at South West of Côte d’Ivoire, which is the major cocoa producing country in the world located in West of Africa). Only healthy pods (pods not rotten) were selected for this study after sorting of harvested pods. Raw cocoa beans was obtained after stages of pod breaking, fermentation and drying. Polyhexamethylene guanidine hydrochloride (PHMGH) disinfectant obtained from the Department of Biological Sciences of Saint-Boniface University (Canada) was used for the treatment of cocoa beans.
2.2. Raw Cocoa Beans Sampling Steps
- - Cocoa pod breakingThe opening of the pods was carried out with a wooden club. The fresh beans were stripped of their placentas were then sorted to remove defective beans and retain only the healthy.- FermentationThe fermentation was conducted in three practices and lasted 6 days without stirring the beans: (i) piled on banana leaves, (ii) in wooden box and (iii) in plastic box. For each practice, a batch of 56.25 kg of fresh beans have been fermented. The boxes used each measure 50x35x30 cm3.- Processing of cocoa beans with the PHMGHAfter 6 days of fermentation, each batch (56.25 kg) of fermented beans was partitioned into three equal parts. One of the units (18.75 kg) was dipped in a solution (5 L) of PHMGH at 2 mg/mL and another in a solution (5 L) of PHMGH at 4 mg/mL for 15 min. The third part has not undergone treatment with PHMGH and was considered as control sample. In total, nine parts of 18.75 kg of fermented beans were formed.- DryingEach of the nine parts of 18.75 kg of fermented beans having undergone or not the disinfection treatment was subdivided again into three equal fractions of 6.25 kg of fermented beans each. The three fractions each of nine parts of 18.75 kg of fermented beans were dried in the sun in three different ways: on a plastic tarp, on a cemented area, and on a grid. The surface used for each drying method was 3x2 m2. Solar drying lasted 6 days (8 am until to 5 pm) for three different methods. The beans are brewed 8 times a day during drying due to a brewing per hour. The specific treatment of the cocoa according to the technological parameters, yielded 27 samples consisting of about 3 kg of raw cocoa beans (fermented and dried cocoa) each.- StorageEach of the 27 raw cocoa beans samples obtained was divided into three parts of about 1 kg of beans packed in small jute bags. One of three parts for each of the 27 samples was transferred (T=0) in the laboratory (in Abidjan) for analysis. The other 2 units were the object storage (conservation) in the house (store) of the producer, where the latter usually stores its product. After one month of storage for each sample, one of the two stored units were collected for laboratory analysis. The third part of each of the 27 samples was analyzed in the following month. Figure 1 shows the main raw cocoa beans sampling stages.
 | Figure 1. Major raw cocoa beans sampling steps |
2.3. Physico-chemical Analyses
- × Moisture content of cocoa beansMoisture in the beans was determined using the International Organisation for Standardization (ISO) method [27]. Approximately 10 g of ground bean sample was placed into a pre-weighed dish (W1) with a lid and re-weighed to the nearest mg (W2). The dish and contents were put in an oven at 103±2°C for 16 h. The lidded dish was then removed from the oven and put in desiccators to cool. After cooling they were weighed (W3) and the percentage of moisture content was calculated using the following equation:
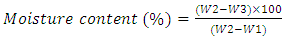 | (1) |
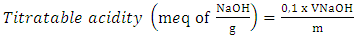 | (2) |
2.4. Fungal Analyses
- Ten grams of each sample coarsely ground cocoa with a brand stomacher AES (France), were added to 90 mL of BPW (EFA) in a sterile stomacher bag (France). The mixture was created by mixing for homogenization for 1 minute. The suspension was considered the mother suspension. Then serial dilutions were made from 10-2 to 10-6 in 9 mL of sterile buffered peptone water.To estimate the population in molds, surface inoculation method was used. This method allows molds to have the same access to oxygen and uniform development of the colonies. To this, 0.1 mL of each dilution was spread evenly over the Dichloran Rose Bengal Chloramphenicol agar (DRBC agar), and for each dilution 2 Petri dishes were seeded. The Petri dishes were then incubated at 30°C for 3 days.The colonies were counted and the results were expressed as colony forming units (CFU) per gram of cocoa. The number N (population estimate of mold) was calculated using the equation [29]:
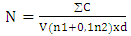 | (3) |
2.5. Statistical Analyses
- The results were the subject ANOVA with the Statistica 7.1 software. In case of significant difference between the studied parameters, the ranking of means was done according to the test of Newman-Keuls. The significance level is α = 0.05.
3. Results
3.1. Prevalence of Fungal Contamination of Processed Raw Cocoa Beans with PHMGH
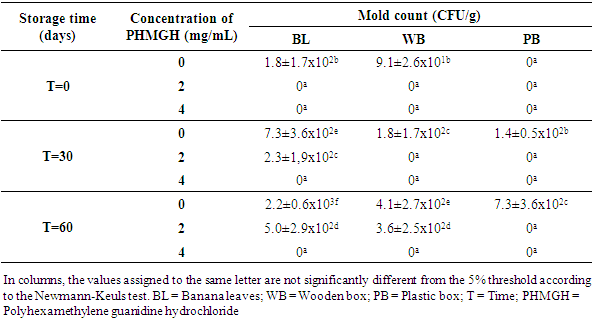
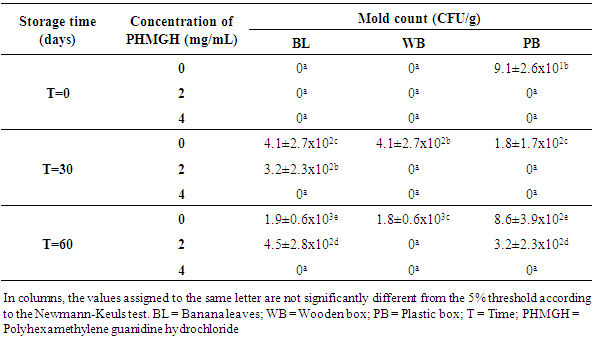
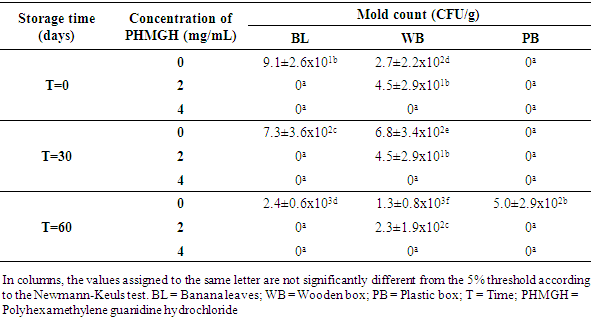
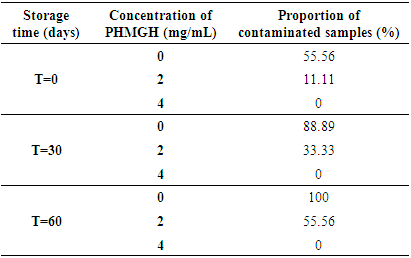
- Table 1 shows the evolution of the load in mold of cocoa beans dried on cemented area, during storage at the producer, according to the methods of fermentation and concentrations of PHMGH. At time T=0, the number of mold in the control samples (samples without PHMGH) was 1.8x102 CFU/g for sample fermented in banana leaves, 9.1x101 CFU/g for sample fermented in wooden box. No mold was detected in the control sample fermented in plastic box. Cocoa samples treated with 2 and 4 mg/mL of PHMGH showed no mold contamination at the time T=0.
|
|
|
|
3.2. Physical and Chemical Characteristics of Cocoa Beans Treated with Disinfectant
3.2.1. Moisture Content
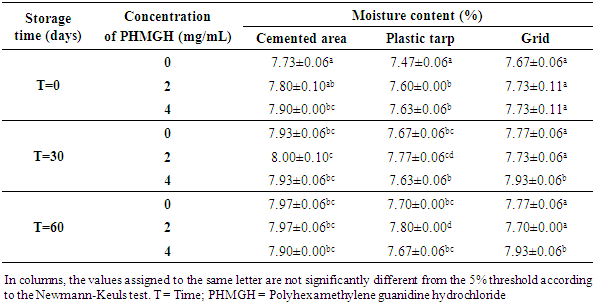
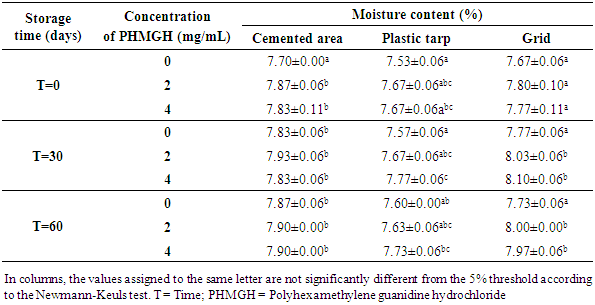
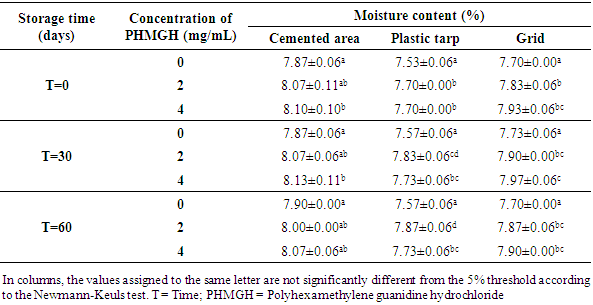
- Table 5 shows the variation in moisture content of cocoa beans fermented in banana leaves during storage, depending on drying surfaces and PHMGH concentrations. At the end of drying, before storage, the moisture content of the cocoa beans ranged from 7.73 to 7.90% for samples dried on cemented area, from 7.47 to 7.63% for samples dried on plastic tarpaulin and 7.67 to 7.73% for those dried on grid. The moisture content of the samples treated with the PHMGH was slightly higher than that of the controls. At the end of 60 days storage time, the moisture content of various samples of cocoa beans had slight variations. However, for each drying surface, there is no significant difference (p>0.05) between these variations regardless of the concentration of PHGMH.
|
|
|
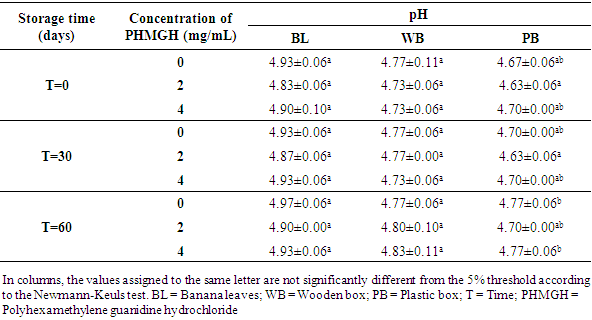
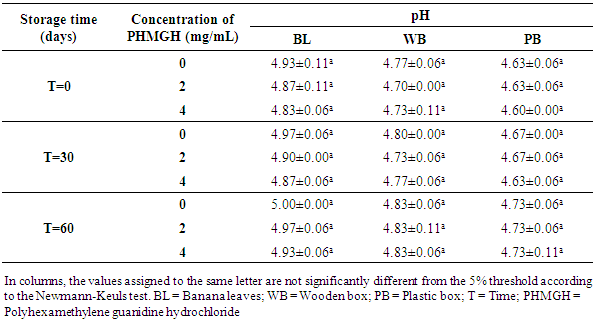
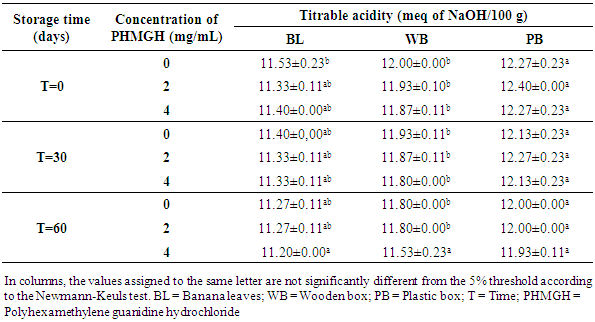
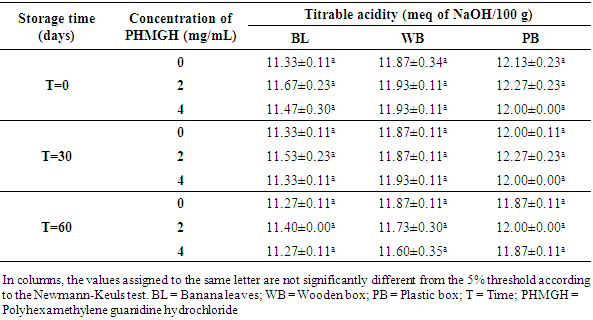
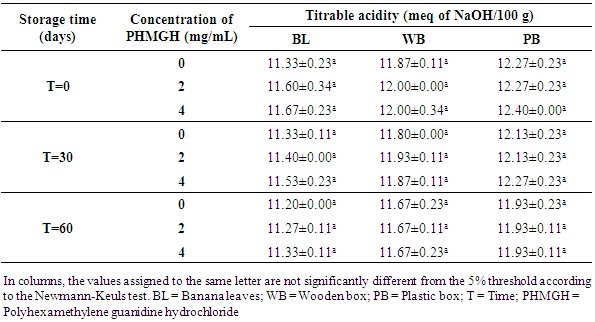
3.2.2. pH and Titratable Acidity
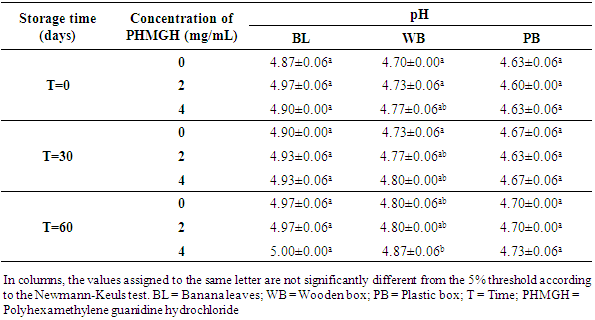
- Table 8 shows the pH variation of the cocoa beans treated with PHMGH and dried on cemented area, during storage, depending on fermentation methods. Before storage, the pH of cocoa beans samples fermented in banana leaves was between 4.87 and 4.97, between 4.70 and 4.77 for samples fermented in wooden box and between 4.60 and 4.63 for those fermented in plastic box. For each method of fermentation, the pH of the cocoa beans does not vary significantly (p>0.05) from one concentration of PHMGH to another. At the end of the storage time, the pH of cocoa beans rose slightly but not significantly (p>0.05).
|
|
|
|
|
|
4. Discussion
- The prevalence of fungal contamination at the pilot scale and the characterization of some physicochemical parameters of cocoa beans treated with Polyhexamethylene Guanidine Hydrochloride (PHMGH) were determined during storage. The physicochemical characterization showed that after six days of drying, the moisture content of the raw cocoa beans samples were lower in the control samples (untreated with PHMGH) compared to those treated with solutions of PHMGH. This difference, which results in a delay in the drying of the beans treated with PHMGH, could be explained by the absorption of water used for the treatment. The moisture content of the raw cocoa beans also made it possible to note that, whatever the fermentation method used, the drying of the cocoa beans on plastic tarpaulin was accelerated more than that effected on grid and cemented area. Throughout the shelf life, the moisture content of most samples remained below the critical value of 8%, beyond which there may be an abundant proliferation of molds [27, 28]. The few variations in the moisture content of the samples observed during storage are in agreement with those made by Hamid and Lopez [27]. For these authors, the beans change moisture according to the place of storage. Therefore, properly dried cacao beans could absorb moisture from the air during storage. This would imply a moisture gain for the raw cocoa beans as supported by Guéhi et al. [9].The mold count revealed that PHMGH is effective in the control of fungal contaminants in raw cocoa beans. Indeed, no sample treated with 4 mg/mL of PHMGH has been contaminated with molds. The few samples treated with 2 mg/mL of PHMGH, contaminated, had significantly lower loads than controls, thus confirming the work of Yao et al. [25]. These authors noted from their in vitro experiments that PHMGH, from 1.9 mg/mL, exerts fungicidal activity on molds: Absidia corymbifera, Rhizopus oryzae, Aspergillus tubingensis, A. tamarii, A. flavus and Penicillium chrysogenum, isolated from raw cocoa beans. Furthermore, Koffi-Nevry et al. [26] recorded PHMGH antifungal activity against Mucor sp., Botrytis sp., Penicillium sp., Geotrichum sp., Aspergillus sp., and Colletotrichum sp., fungi often responsible for the deterioration of papaya. The slight increase in moisture observed in the samples treated with 4 mg/mL of PHMGH compared to that of the control samples did not allow the fungi to develop on these samples. This means that the PHMGH exerts a fungicidal activity in the raw cocoa beans at this concentration. On the other hand, for the concentration of 2 mg/mL, PHMGH can be considered as a fungistatic agent since it limits or blocks the growth of fungal contaminants in cocoa beans. Thus, the growth of these contaminants resumes after the time of inhibition caused by the disinfectant.The highest mold load in the order of 103 CFU/g of samples not treated with PHMGH is consistent with the results of Guéhi et al. [9]. Indeed, these authors found of the order of 103 CFU/g as molds load of the brown beans samples and of the order of 106 CFU/g that of the black and clustered beans samples, in a comparison study of loads of fungal contaminants in different types of raw cocoa beans (brown, black and clustered beans) collected from producers. Cocoa beans that were the subject of the present study are brown beans; rotten, black and clustered beans having been eliminated since cocoa pods breaking. According to several authors whose Oyeniran [30] and Barel [31], these defective beans are very susceptible to mold growth during fermentation and drying.The measurement of pH and titratable acidity of raw cocoa beans showed the influence of fermentation methods on these parameters. Thus, the beans fermented in plastic box were more acidic at the end of drying than those fermented in wooden box and in banana leaves. However, there is no significant difference (p>0.05) between the pH values of the control samples and those of samples treated with the PHMGH according to fermentation method practiced and storage time. This confirms the neutrality of the PHMGH underlined by Oulé et al. [24]. Titratable acidity and pH results obtained in this study are similar to those of Guéhi et al. [32]. These authors found values between 4.73 and 5.66 for pH and between 10.60 and 23.60 meq NaOH/100 g for titratable acidity of raw cocoa beans in similar studies. They also demonstrated that variations in pH and titratable acidity of raw cocoa beans are related to the fermentation conditions including the method and the fermentation time.According to several authors [20, 21, 33, 34], fermentation of cocoa beans takes place in two phases: an anaerobic phase and an aerobic phase. During the anaerobic phase, the lack of oxygen is due to the crowding of beans. Due to the high content of fermentable sugars and the acidic pH of the pulp, the cocoa mass is rapidly contaminated by yeasts from the fermentation equipment and from the hands of operators and the environment. As their number quickly became very important, the oxygen present in the cocoa mass was consumed. Thus, the yeasts transform the sugars contained in the cocoa pulp into alcohol (ethanol) with a release of carbon dioxide. Between 16 and 48 hours of fermentation, while the lactic acid bacteria grow in these anaerobic conditions, produce lactic acid, the number of yeast decreases [20, 35, 36, 37]. The aerobic is created by the gradual disappearance of the mucilaginous pulp surrounding the beans under the action of yeasts and lactic acid bacteria. According to several authors whose Adeniyi et al. [21], brewing the beans during fermentation is an essential step in the aeration of beans. Thus, acetic bacteria in the presence of oxygen contribute to the fermentation by oxidizing the ethanol produced upstream by yeasts to acetic acid which is very volatile during solar drying [38-40]. Unlike acetic acid, which is highly volatile, lactic acid has less volatile properties [38].During fermentation in banana leaves, the beans would be sufficiently aerated, which would encourage abundant growth of acetic bacteria. The low acidity of the samples fermented in banana leaves compared to the others could therefore be explained by the acetic acid which had to volatilize during solar drying. On the other hand, the relatively high acidity of samples fermented in box, mainly plastic, is due to the poor aeration of the beans as supported by Guéhi et al. [41]. Anaerobic lactic acid bacteria probably produced a significant amount of lactic acid that could not volatilize during drying.
5. Conclusions
- Treatment of cocoa beans with PHMGH for the control of fungal contaminants in raw cocoa beans yielded satisfactory and variable results depending on the concentration. Thus, PHMGH exerted a fungicidal activity at the concentration of 4 mg/mL in raw cocoa beans while at 2 mg/mL, a fungistatic effect was observed. It should be noted that raw cocoa beans treated with PHMGH retains its physicochemical characteristics, namely moisture content, pH and titratable acidity whatever the fermentation method and the drying surface. PHMGH, a non-toxic disinfectant for humans, can therefore be used at a concentration of 4 mg/mL for the preservation of cocoa beans.
ACKNOWLEDGEMENTS
- We thank all cocoa producers who welcomed us and helped us during the realization of this study. Brave producers, receive here the expression of our highest gratitude!
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML