-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2017; 7(1): 1-6
doi:10.5923/j.fph.20170701.01

Occurrence of Bisphenol A (BPA) in Ponds, Rivers and Lagoons in South-Western Nigeria and Uptake in Cat Fish Evidence of Environmental Contamination
Tunmise Makinwa, Patrick Uadia
Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Nigeria
Correspondence to: Tunmise Makinwa, Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

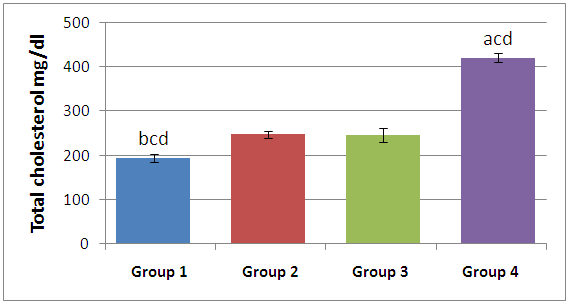
Fish, being one of the most abundant groups in the aquatic ecosystem are sometimes exposed to all sorts of environmental assaults such as heavy metal pollution and agricultural chemicals contamination, which can consequently have serious effects on the nutritive quality of fish inhabiting the contaminated aquatic environment. This study investigated the level of Bisphenol A (BPA) (a known endocrine disruptor) in the serum of Cat fish (Clarias gariepinus and Clarias nigrodigitatus) obtained from different ponds, rivers and lagoons in south western Nigeria, using the indirect competitive enzyme-linked immunosorbent assay (ELISA) method. The limit of detection (LOD) of the kit was 10pg/ml. BPA was found in all fish samples caught (ranging from 0.05±0.01µg/L- 2.76±0.97µg/L), with pond raised fishes having the highest BPA concentrations. Analysis of serum total cholesterol levels in the Cat fish revealed that Cat fish caught from lagoons had a significantly (p<0.05) higher serum total cholesterol levels compared with the others, no correlation was found between serum total cholesterol and serum BPA concentration in the fishes. The study also revealed there was no significant association between BPA concentrations (6-52µg/L) in the water samples and the physicochemical properties of the water samples from which the fishes were obtained. Contamination from aquatic environment is a major source of BPA exposure in fishes, hence there is need for regular monitoring of our aquatic environment and effective methods of waste disposal should be adopted to reduce the level of contaminants in these aquatic environment.
Keywords: Bisphenol A, Cat fish, Aquatic environment, South-western Nigeria
Cite this paper: Tunmise Makinwa, Patrick Uadia, Occurrence of Bisphenol A (BPA) in Ponds, Rivers and Lagoons in South-Western Nigeria and Uptake in Cat Fish Evidence of Environmental Contamination, Food and Public Health, Vol. 7 No. 1, 2017, pp. 1-6. doi: 10.5923/j.fph.20170701.01.
Article Outline
1. Introduction
- Many of the thousands of anthropogenic chemicals currently released into the environment are endocrine-disrupting compounds (EDCs) [1]. These are defined as exogenous chemicals or chemical mixtures that impact endocrine system structure or function and cause adverse effects [2]. Chemicals implicated as having endocrine disrupting effects include; biocides, industrial compounds, surfactants, and plasticizers which include Bisphenol A (BPA) [3-7]. Bisphenol A (BPA) is a chemical that has been attracting increased attention because of its high potential for human exposure. Bisphenol A (BPA) is used as the base compound in the manufacture of plastics [1]. It is also used in multitude of products including food and beverage packaging, flame retardants, adhesives, building materials, electronic components, and paper coatings [8]. Worldwide, over 6 billion pounds of BPA are produced each year and over 100 tons are released into the air annually [1]. BPA has been shown to leach from food and beverage containers (via hydrolysis of polycarbonate plastics and epoxy resins) [9], dental sealants and composites [10] under normal conditions of use [5], these have been the major sources of human exposure. While the source for human exposure to BPA is food and liquid storage containers, BPA is released into the environment through either sewage treatment effluent (via human-ingested BPA being eliminated through sewage; [11]), landfill leachates (via hydrolysis of BPA from plastics; [12]), or natural degradation of polycarbonate plastics. While sewage effluent and landfill leachates are point sources of BPA in the environment, fragments of epoxy resins and polycarbonate plastic debris entering the watershed through runoff are non-point sources.Pharmacologically BPA is a hormonally active agent with well documented estrogenic activities [13, 14]. Data from multiple sources indicated that the amount of BPA to which humans are exposed may cause adverse health effects; this has raised concerns among regulatory agencies all over the world, because of its estrogenic properties and the conserved role that estrogen plays in regulating human and animal physiology and pathophysiology [15, 16]. Whatever the mechanism, studies have shown that BPA can cause effects in animal models at doses in the range of human exposures, indicating that it can act at lower doses than predicted from some in vitro and in vivo assays [17, 18]. More than 150 published studies describe BPA effects in animals exposed to < 50 mg/kg/day, including altered development of the male and female reproductive tracts, organization of sexually dimorphic circuits in the hypothalamus, onset of oestrus cyclicity and earlier puberty, altered body weight, altered organization of the mammary gland, and cancers of the mammary gland and prostate; > 40 of these studies examined doses less than the reference dose (RfD) [19]. All these studies that have examined the effects of BPA are either in vitro studies or have been conducted on rats and mice, but the past few years have seen a dramatic increase in research examining BPA exposure on selected invertebrates, fishes, amphibians, reptiles, birds, and wild mammals. Many authors have examined the level and the effect of exposure to BPA in aquatic organisms all over the world. This study compared the level of BPA in cat fish caught from different aquatic habitats in south-western Nigeria, with the aim of determining the association between the sources/level of BPA exposure in these water bodies and the uptake of BPA by the fishes. The association between serum BPA and serum cholesterol in the fishes was determined.
2. Materials and Methods
2.1. Experimental Animals
- Thirty (30) cat fishes (Clarias gariepinus and Clarias nigrodigitatus) were caught from ten (10) different locations in South-western Nigeria. The sampling sites were chosen to reflect different activities in the environmentGroup 1: Fish (n=9) samples were obtained from 3 domestic/commercial hatchery (concrete pond) at Owo (Ondo State) and Ebute-meta (Lagos State) and brought to the laboratory in kegs. The pond is supplied with bore hole water stored in plastic storage tanks.Group 2: Fish (n=6) samples were obtained from 2 earthen ponds at Owo town and Akure town (Ondo State) and were brought to the laboratory in kegs. The pond is supplied with water channelled from nearby stream from the location.Group 3: Fish (n=6) samples were obtained from 2 rivers at Ayede-Ogbese town (Ondo State) and Ebute-meta River in Lagos (Lagos State) they were brought to the laboratory in kegs. The river is the major fishing grounds in the area during the raining and dry season. Group 4: Fish (n=9) samples were obtained from 3 lagoons (Makoko, Ebute-meta and third main land) in Lagos (Lagos State) and brought to the laboratory in kegs. Both domestic and agricultural wastes are dumped into the water body; the river is also a runoff point for many industrial effluents.
2.2. Blood Samples Collection
- The live fishes (3 from each location) weighing between 200-700g were dissected and the blood samples were obtained for the determination of serum Bisphenol A and total cholesterol. Blood was collected in plain bottles to obtain serum. The samples were centrifuged and the sera were stored at −20C.
2.3. Determination of the Physicochemical Characteristics of the Water Samples
- The physicochemical properties (pH, electrical conductivity, turbidity, total alkalinity, total acidity, Chloride concentration, total hardness, Dissolved Oxygen (DO), Biochemical oxygen demand (BOD)) of the pond, stream, river and water lagoon samples were determined using standard methods described by the Association of official analytical Chemists [20].
2.4. Determination of Total Cholesterol
- The cholesterol in the serum of experimental fish was determined following the method described in the Randox cholesterol standard kit leaflet (Randox laboratory LTD, UK).
2.5. Determination of Serum BPA Levels in Fish and Water Samples
- BPA concentrations in the serum and water samples were analyzed by enzyme-linked immunosorbent assay (ELISA) method using BPA ELISA kit from Detroit R&D, Inc, Metro Centre for High Technology Bldg. (MCHT) Detroit, USA, with product code Cat # BPA 1.
2.6. Statistical Analysis
- Values are expressed as the mean ± SE. Results were statistically analyzed by one-way analysis of variance (ANOVA) for differences between means of different groups. All data were analyzed using SPSS statistical package (SPSS Inc.) version 13.0. A probability of P<0.05 was considered statistically significant.
3. Results
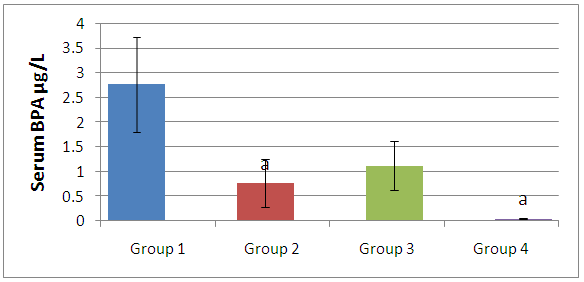
- From the results there were no significant associations between BPA concentration in the water samples and their physicochemical properties.As shown in figure 1, the serum BPA levels of cat fish (Clarias gariepinus) obtained from different sources of concrete ponds was significantly high (p<0.05) compared with those caught from lagoons and rivers, however there was no significant difference in the serum BPA concentration of fishes obtained from concrete ponds and earthen ponds.
4. Discussion
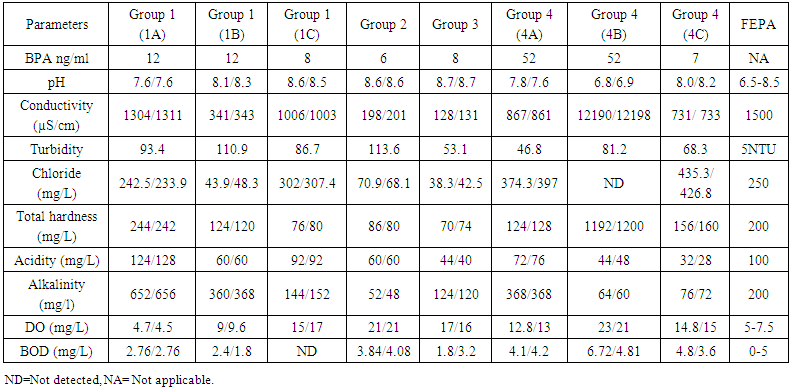
- Rivers, lakes, and estuaries are major sinks for Bisphenol A (BPA). These surface waters accumulate BPA leached from plastic debris and landfill wastes along with BPA-containing sewage and effluent. In aerobic environments such as most rivers, BPA has an environmental half-life of between 4.5 and 4.7 days [21], being degraded primarily by bacteria [22]. However, BPA has limited biodegradation under anaerobic conditions [23].As shown in table 1, the BPA concentrations in the water samples obtained from different locations varied (from 6-52µg/L) with those obtained from lagoons having the highest concentration of BPA. No association was observed between the physicochemical properties of the water sampled and their levels of BPA. Water samples varied greatly in their concentration of BPA depending on location and time of sampling. For instance, river water sampling and analyses in Germany have found BPA concentrations between 0.016 and 0.41 µg/L [24, 25], in the United States between non-detectable and 12µg/L [26], and in Japan up to 19µg/L [27]. Due to such dramatic variation in environmental water concentrations of BPA, the question arises as to what concentration to consider as a typical environmental exposure for aquatic animals. The Bisphenol A Global Industry Group (a coalition of the American Plastics Council, the Association of Plastics Manufacturers in Europe, and the Japan Chemical Industry Association) reports environmental concentrations of BPA as median concentrations of numerous environmental samplings (http://www.bisphenol-a.org). However it was concluded by Crain et al. [28] that a more relevant exposure concentration is the maximum amount detected at a site. This is because acute exposure to “organizational disruptors” such as BPA can have long-term detrimental consequences if exposure happens during critical windows of development.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML