-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2016; 6(6): 157-164
doi:10.5923/j.fph.20160606.02

Supercritical Extraction of Cobia (Rachycentron canadum) Liver Oil as a New Source of Squalene
Débora Nascimento e Santos, Eliane Hissae Takahashi, Alessandra Barros Verde, Alessandra Lopes de Oliveira
Food Engineering Department, Faculty of Animal Science and Food Engineering, University of Sao Paulo, Pirassununga, Brazil
Correspondence to: Alessandra Lopes de Oliveira, Food Engineering Department, Faculty of Animal Science and Food Engineering, University of Sao Paulo, Pirassununga, Brazil.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Cobia is a fish cultivated worldwide and there are few studies on the potential of their waste, such as the liver, it's rich in oil and active compounds. The objective of this study was to extract cobia liver oil (CLO) using supercritical CO2 under moderate temperature conditions (50, 60 and 70 °C, 250 bar) and characterize the oil (physico-chemical analysis, fatty acids profile and squalene content). The results showed that CLO is rich in MUFA and presented squalene, suggesting that this matrix can be applied in the production of high nutritional value oil.
Keywords: Liver fish oil, Cobia, Supercritical extraction, Omega 3, Squalene
Cite this paper: Débora Nascimento e Santos, Eliane Hissae Takahashi, Alessandra Barros Verde, Alessandra Lopes de Oliveira, Supercritical Extraction of Cobia (Rachycentron canadum) Liver Oil as a New Source of Squalene, Food and Public Health, Vol. 6 No. 6, 2016, pp. 157-164. doi: 10.5923/j.fph.20160606.02.
Article Outline
1. Introduction
- Cobia, Canadum Rachycentron, is a marine fish native to Brazil and found in tropical and subtropical seas. In captivity, this fish presents a high growth rate with low incidence of disease and produces high-quality meat [1, 2]. Because of these attributes, this species has been widely used in aquaculture in some countries in Asia and the Pacific region, generating a product of great economic value [3]. In Brazil, the production of cobia in captivity was initiated in 2006, and it continues in expansion due to encouragement from the Fishery Ministry and the private sector [4]. The cobia growing in captivity generated stimulus for studies about feeding and management techniques for growth efficiency.The world production of fish, including fishing and fish farming, was 158 million tons in 2012. Out of this total, 136.2 million tons (86% of total production) were used for human consumption, and the remaining 14% (21.7 million tons) was used for non-food purposes such as fishmeal and fish oil production [5]. Fish oil extraction can be classified into physical, chemical, and biological processes. The chemical extraction, realised with organic solvents, is a process well established. However, the use of toxic solvents can result in protein denaturation and loss of functional properties [6, 7]. In this context, supercritical fluid extraction is a potential alternative for the many advantages that it presents in relation to other methods.Supercritical fluid extraction using CO2 as a solvent can be applied to obtain fish oil rich in unsaturated fatty acids at low temperatures. Supercritical CO2 extraction produces no oxygen in the process, which is responsible for the oxidation of fatty acids. The supercritical CO2 density can be easily changed with temperature and pressure alteration, which modifies the solvating power of the solvent. Another known characteristic is the low toxicity of CO2 that is recognised as safe (GRAS) and still produces clean waste extraction and solvent-free extracts [8, 9].The first studies of the application of supercritical CO2 extraction in fish oils were conducted in the 1950s. They studied the phase equilibrium data and compounds of the fish oils obtained by supercritical CO2 and other organic solvents [10]. Some studies have shown the fractions purification of substances such as ethyl esters, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [11], acid esters of fish oils [12], and contaminants [13]. In recent studies, supercritical extraction has been applied for new fish species or by-products [14] or as an extraction method combined with other processes such as molecular distillation [15] in order to optimise the fractionation of compounds with biological activity such as alkylglycerols and squalene.The objective of this research was to obtain cobia liver oil (CLO) using supercritical extraction in different operational conditions in order to characterise this oil by determining physico-chemical parameters and the fatty acid profile and by investigating the presence of bioactive compounds such as squalene. In addition to the study of clean technology to extract CLO, this study aimed to use waste from the fishing industry, such as raw material, for the production of oil with active properties.
2. Materials and Methods
2.1. Materials and Sample Characterisation
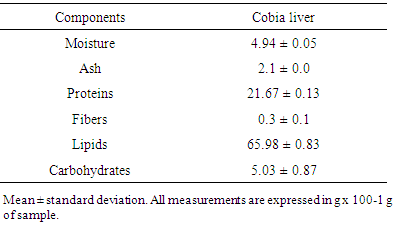
- Frozen cobia (Canadum Rachycentron) liver samples were provided by a commercial marine farm located in Ubatuba/Sao Paulo, BR. The livers were cut into small pieces and freeze-dried for 24 h (LC-1500 Terroni, Sao Carlos, BR). After lyophilisation, they were stored at -20°C until the supercritical extraction. An aliquot of the liver samples was used for chemical composition analysis. Moisture, ashes, lipids, fibre, protein, and carbohydrate assays were performed in triplicate, in accordance with the standards of the Institute Adolfo Lutz (IAL) [16].
2.2. Supercritical CO2 Extraction of Cobia Liver Oil
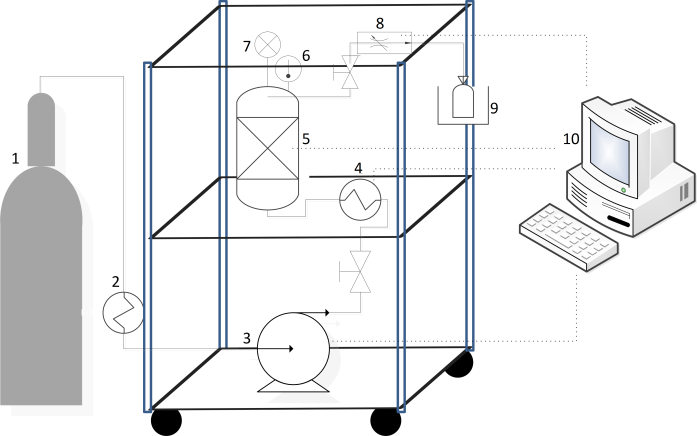
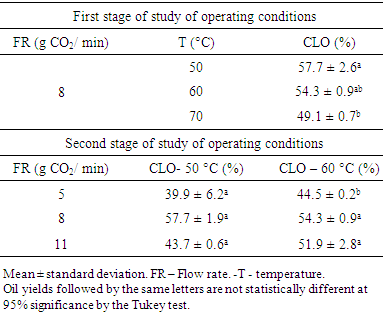
- Lyophilised liver samples (15 g) cut into non-uniform small pieces were placed in a fixed bed extractor with 300 cm³, which was completed with glass beads (with diameter of 5 mm). The equipment used is schematised in Fig. 1 (Thar SFC, Pittsburgh, USA). During operation of the equipment, CO2 (1) (99.9% Oxinitro, Pirassununga, BR) leaves the cylinder and follows to be cooled in a thermostatically controlled bath (2) to -2.3°C (Thermo Scientific, Walthan, USA). Liquid CO2 is pumped with an HPLC pump (3) (Thar P54, Pittsburgh, USA) to a heat exchanger (4), where it is heated to a temperature 5°C lower than the operating temperature.
|
2.3. Physico-Chemical Characterisation of CLO
- Cobia liver samples before and after the supercritical extraction were analysed by scanning electron microscope (Hitachi, TM 3000, Tokyo, JP) for morphological analysis. Sample viscera were fixed with carbon double-sided adhesive in aluminium support, and micrographs were generated with an accelerated voltage of 5 kV.The refractive index (RI), acidity index (AI), peroxide value (PV), iodine value (IV), and saponification number (SN) were quantified in CLO samples according to standardised analytical methods of IAL [16]. The RI was measured in a refractometer (LAMBDA 2WAJ, ATTO Instruments Co, Tsuen Wan, HK) at 25 and 40°C (Zenebon et al. 2008).To determine the AI, we used the titration method, with a standardised solution of sodium hydroxide (Haloquímica, São Paulo, BR) 0.01 M and phenolphthalein as an indicator (Synth, São Paulo, BR). In the PV analysis, CLO was mixed with a solution of acetic acid (Synth Sao Paulo, BR): chloroform (Êxodo, Sao Paulo, BR) (3:2) and a saturated solution of potassium iodide (Synth Sao Paulo, BR). Titration of this solution mixed with distilled water with 0.1 M of sodium thiosulfate (Haloquímica, São Paulo, BR) was made using 0.5 mL of 1% starch (Haloquímica, São Paulo, BR) as a turning-point indicator. In this analysis, a sample with no treatment was titrated and used as a blank.In determining the IV, CLO samples were mixed with cyclohexane (Synth, São Paulo, BR) and Wijs solution (Haloquímica, São Paulo, BR), and after 30 min, potassium iodide (15%) and distilled water were added. The titration was performed with a standardised sodium thiosulfate solution, using starch as an indicator. A blank solution without oil was titrated under the same conditions. The SN was determined by adding an alcoholic solution of potassium hydroxide (4%) (Exodus, São Paulo, BR) to the CLO, and this solution was then heated for 30 min. The mixture was titrated with a standardised solution of hydrochloric acid (Haloquímica, São Paulo, BR), using phenolphthalein as an indicator. A control solution without oil was made and titrated under the same conditions.
2.4. Fatty Acids Profile and Squalene Quantification
- The fatty acids profile was performed according to the method 991.39, AOAC [18], with modifications. In a Falcon tube containing 50 mg sample of CLO, 4 mL of NaOH solution in methanol (Synth, Diadema, BR) 0.5 M was added, and the mixture was heated at 100°C in a water bath for 5 min. Then, 5 mL of methanolic solution of BF3 (Sigma-Aldrich, Saint Louis, USA) was added and heated for 30 min. Subsequently, 4 mL of saline solution and 5 mL of hexane (Merck, Darmstadt, DE) were added, and the mixture was placed under stirring for 1 min. After phase separation, the light phase, containing the methyl esters of fatty acids, was transferred to a flask and dried. The mass of methyl ester fatty acids was dissolved in hexane at a known concentration and transferred to a vial.The fatty acids analysis was performed by gas chromatography coupled with mass spectrometry, GC/MS (QP 2010 Plus, Shimadzu, Tokyo, JP) with an automatic injector (AOC-5000, Shimadzu, Tokyo, JP), according to the method 996.06, AOAC [18], with modifications. In this analysis, we used a capillary column with stationary phase poly (biscyanopropyl siloxane) (100 m × 0.25 mm id × 0.20 µm df, SP–2560) (Supelco, Bellefonte, USA), gas helium as a carrier gas to an internal speed of 19.5 cm/s. It was injected 1.0 µL of the solution containing methyl esters in hexane in the splitless mode. The injector temperature was 250°C, and the detector was 260°C. The oven temperature was regulated to obtain a ramp starting at 100°C for 1 min, 100°C to 195°C at a rate of 5°C/min, and then 195°C to 250°C. The previous identification of the peaks was made by comparison of the mass spectra from the NIST library and NIST 08S, and the quantification was performed by external standardisation relating the peak area with the standard curve of myristic acid methyl ester (Sigma-Aldrich, Saint Louis, USA).Squalene quantification was performed according to Lu et al. [19]. In a Falcon tube containing 0.5 g of CLO, 40 mL of a mixture of methanol: acetone (7:3, v/v) was added. The mixture was homogenised for 5 min in a mechanical shaker (vortex) and stored at -2°C for 30 h. The supernatant was quickly filtered through a 0.45 mm membrane (Whaltman, Kent, UK). The residue was washed with 5 mL of a cooled solution of methanol: acetone (7:3, v/v) at -20°C. The filtrate was dried in a vial and stored under refrigeration. At the injection into GC/MS (QP 2010 Plus, Shimadzu, JP), the filtrate was resuspended in 5 mL of acetone.The chromatographic analysis was performed according to Brunner et al. [20], with modifications. It was used as stationary phase column RTX-5MS (5% diphenyl/95% dimethyl polysiloxane) (30 m × 0.25 mm id × 0.20 µm df Restek, Bellefonte, USA), helium as carrier gas to an internal speed of 19.5 cm/s. The solution of squalene in acetone (1.0 µL) was injected in splitless mode. The temperatures of the injector, detector, and interface were 280°C, 300°C, and 300°C, respectively. The oven temperature was regulated to obtain a ramp starting at 130°C for 2 min, 130°C to 200°C at a rate of 10°C/ min, from 200°C to 290°C at 5°C/ min and remaining at 290°C for 15 min. The previous identification of the peaks was made by comparison of the mass spectra with those from NIST and 08S NIST library and by comparing the retention time with the standard. The external standardisation was applied in the squalene quantification, relating the peak area of the samples with the standard squalene curve (Santa Cruz, Dallas, USA).The means of the results of physico-chemical analysis and squalene contents were evaluated statistically by ANOVA, and means differences were tested by Tukey’s test, for 95% of significance level.
3. Results and Discussion
3.1. Cobia Liver Characterisation and Extraction Yield
- Cobia liver is a rich source of lipids, which comprise over 65% of the total. It also presents a significant amount of protein (Table 2). The lyophilisation process reduced water content and provided lipids (macromolecular group most abundant in the viscera after water) for extraction.
|
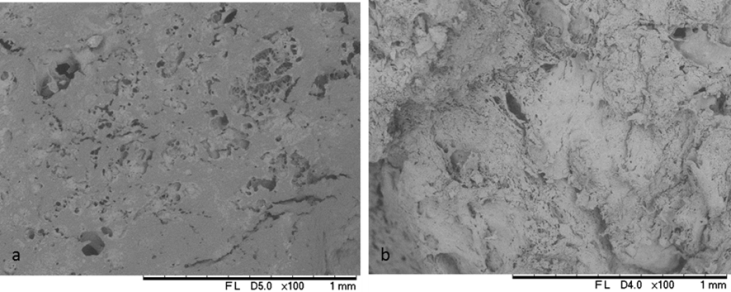 | Figure 2. Scanning electron microscopy of freeze-dried cobia liver before the supercritical extraction of oil (A) and after extraction to 250 bar, 8 g CO2 / min, 60°C (B) |
3.2. Cobia Liver Oil
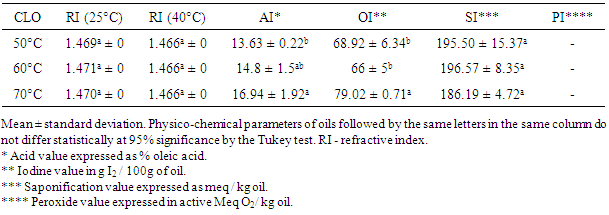
- RI values at 25 and 40°C did not vary (p < 0.05) when different temperatures were used in the extraction (Table 3). These RIs are similar to other fish oils, such as the crude oil from Oreochromis niloticus and Pseudoplatystoma corruscans × P. fasciatum [25]. According to a study by Immanuel et al. [26], oil from Sufflamen capistratus liver obtained by different extraction methods showed no significant differences in the RI. The authors explained that the refraction angles are defined as a function of the fat composition, and a variation in these values could indicate that it was made a mix of different oils. RI is an index that indicates the quality standard of oils and fats.
|
|
4. Conclusions
- This original research is important for the fish chain, including cultivation and processing, since this study shows a possibility to increase the CLO production as a by-product with active properties. The data showed here insert the cobia liver in the class of the great producers matrices of fish oil rich in squalene. CLO extracted with supercritical CO2 is an oil with physico-chemical characteristics similar to other fish oils marketed with a fatty acid profile showing high levels of MUFA and large amounts of omega 3. CLO also showed a high content of squalene, an important bioactive compound, which encourages the application of this oil not only in the food industry but also in the pharmaceutical industry.
ACKNOWLEDGEMENTS
- The authors would like to thank FAPESP (São Paulo Research Foundation, Brazil) for the financial support under the research projects 2012/00467-3 and for the scholarship for the doctoral student. The authors would also like to thank Dr Sheyla Vargas, who donated the cobia liver, and Terroni Company (São Carlos, Brazil), which lyophilised the liver samples.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML