-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2016; 6(4): 93-98
doi:10.5923/j.fph.20160604.02

High Prevalence of Antibiotic Residues among Broiler Chickens in Gaza Strip
Abdelraouf A. Elmanama1, Mohammed A. Albayoumi2
1Medical Laboratory Sciences Department, Islamic University-Gaza, Gaza Strip, Palestine
2Veterinary Services, Ministry of Agriculture, Gaza, Palestine
Correspondence to: Mohammed A. Albayoumi, Veterinary Services, Ministry of Agriculture, Gaza, Palestine.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study was carried out to evaluate the presence of some antimicrobial residues in broilers slaughtered in Gaza strip. Three hundred sixty five chicken breast samples were collected from poultry slaughterhouses distributed in the study area. Samples were screened for the presence of β-lactams, aminoglycosides, macrolides and tetracyclines (as groups) using a 7-Plate bioassay method. Chicken carcasses were divided into three categories; category (A); ≤1.5 kg, category (B); > 1.5-2 kg and category (C)>2 kg. A total of 88 (24.1%) samples were positive for one or more of antibiotic residues, (53.41%) of them were from category (A), followed by (32.95%) for category (B) and the least category was group (C) (13.63%). Tetracyclines, aminoglycosides, β-lactams and macrolides residues were detected in 41(43.15%), 26(27.36%), 20(21%) and 8(8.42%) respectively. Results confirmed the presence of antibiotic residues in poultry meat samples. Thus, it is highly recommended to establish a national monitoring program for antimicrobial residues in foods.
Keywords: Antibiotic residues, Broiler, Bioassay, Gaza strip
Cite this paper: Abdelraouf A. Elmanama, Mohammed A. Albayoumi, High Prevalence of Antibiotic Residues among Broiler Chickens in Gaza Strip, Food and Public Health, Vol. 6 No. 4, 2016, pp. 93-98. doi: 10.5923/j.fph.20160604.02.
Article Outline
1. Introduction
- Antimicrobials are generally used in farm animals for therapeutic and prophylactic purposes. They include a large number of different types of compounds, which can be administered either in feed, in drinking water or by injection [1]. Ingesting residues of drugs or their metabolites in meat and other foods of animal origin may cause adverse effects which include; carcinogenicity, mutagenicity, bone marrow toxicity (Chloramphenicol) and allergy (Penicillin) [2]. Antimicrobials residues in food disrupt the intestinal microbiota and increase the development of resistant bacteria in the general population [3]. Drug resistance has gained its importance due to its ability of transmission to other enteric pathogens which have posed serious public health problems [4]. In addition soil microbiota which receives antimicrobial residues (AMR) via birds manure may affect the human health as a source of developing resistant microorganisms [5, 6].The Codex Alimentarius Commission (CAC) established the Codex Committee on Residues of Veterinary Drugs in Food which defined Maximum Residue Limits (MRL) for about fifty nine veterinary drugs [7]. Maximum Residue Limit means the maximum concentration of residue resulting from the use of a veterinary medicinal product (expressed in mg/kg or µg/kg on a fresh weight basis) which may be accepted by the community to be legally permitted or recognized as acceptable in or on a food [8]. Antimicrobial residues are detected by chemical, biological and immunological methods. Detection methods can be classified by their degree of quantification into qualitative, semi-quantitative and quantitative methods. Chicken meat, rather than other foods, such as milk or beef, was chosen for a variety of reasons; one of them is that poultry meat is largely consumed in Gaza strip by consumers of all ages. To the best of our knowledge, there is not any previous study concerning antimicrobial residues in meat, so we carried this first study in an attempt to tackle this issue in Gaza strips. The aim of this study is to determine the presence of four antibiotic group residues in broilers slaughtered in Gaza strip.
2. Materials and Method
2.1. Materials
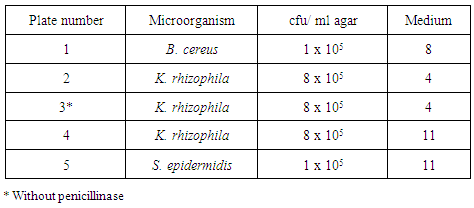
- 2.1.1. Assay microorganisms: Lyophilized microorganism pellets of Bacillus cereus spores ATCC 11778, Kocuria rhizophila cells ATCC 9341a, Staphylococcus epidermidis cells ATCC 12228 were from (KWIK-STIK™, Microbiologics, USA). 2.1.2. Antibiotics and assay media: Sensitivity antibiotic disks Tetracycline Te (30), Penicillin P (10), Erythromycin E (15) and Neomycin N (5). Assay Media No 4, 8 and 11 were purchased from Himedia (India).2.1.3. Others; Bioassay cylinders were from (HiMedia, India). Penicillinase from (BD, USA). Monobasic Potassium phosphate, dibasic Potassium phosphate from (Liofilchem, Italy), and Whirl-Pak bags (Nasco, USA).
2.2. Study Area
- The study included the five governorates of Gaza strip; North Gaza, Gaza, the Middle, Khanyounis and Rafah. The study area was divided into 3 regions: 1. North Gaza and Gaza, 2. The Middle area and 3. Khanyounis and Rafah.
2.3. Sample Collection
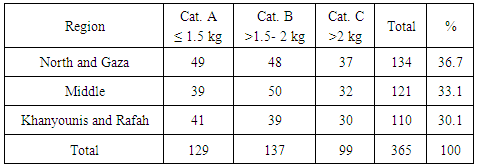
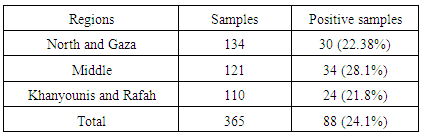
- Total of 365 chicken breast muscle samples were collected from different slaughterhouses distributed in the study area (Table 1). In addition, samples were collected at intervals to ensure sample representativeness during the period extended from January to June 2014. Samples were packed in sterile bags and kept in a deep freezer (-22°C) at biological sciences department, Islamic University-Gaza until analysed. Carcasses were divided into three categories according to their weights; category A; 1-1.5 kg, category B; > 1.5-2 kg and category C>2 kg. From each carcass, 20 gm of breast meat were collected as a sample.
|
2.4. Principle of the Test
- The principle of the test is preparing plates seeded with sensitive bacteria at specific conditions that can presumptively indicate the presence of specific antimicrobial group residues depending on the presence or absence of inhibition zones on the seeded plates.
2.5. Petri Plates Preparation
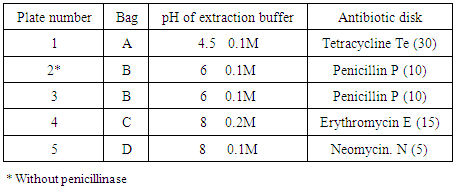
- Lyophilized Bacillus cereus spores (ATCC 11778), Kocuria rhizophila cells (ATCC 9341a) and Staphylococcus epidermidis cells (ATCC 12228) were cultured according to manufacturer's instructions. Briefly, after hydration of the crushed pellets in the tube and saturation of the included cotton swabs of the kit, swabs were gently rolled into Blood Agar plates then were streaked using sterile loop to obtain isolated colonies. Three types of Antibiotic Assay Medium No (4, 8 and 11) were prepared according to manufacturer's instructions (HiMedia, India). Five bottles were prepared; two bottles of Medium No 4, two bottles of Medium No 11 and one bottle of Medium No 8. After autoclaving, media bottles were placed in a water bath at 48°C until they cooled to water bath temperature. The required quantity of each freshly prepared bacterial suspension was added into the prepared Antibiotic Assay Media to make a final concentration of bacteria as recommended by (FSIS) protocol; 5 x 103 cfu/ml for B. cereus into Medium No 8, concentration of 8 x 105 cfu/ml for K. rhizophila into two bottles of Medium No 4 and into one bottle of Medium No 11 and a concentration of 1 x 105 cfu/ml for S. epidermidis into Medium No 11. Penicillinase was added to all bottles except for Medium No 4 containing K. rhizophila. Bottles were shaken well then six ml of the contents were transferred into Petri plate (90 mm) to make a layer thickness of 1 mm; plates were gently swirled to ensure uniformity. Prepared plates were stored at 2 – 8ºC and used within 5 days after preparation) [9]. Plates were numbered from one to five as shown in (Table 2).
|
2.6. Sample Preparation and Storage
- Samples were kept cold at all times. They were allowed to remain briefly at room temperature only during processing and testing. Each sample was divided into four portions (approximately five grams a piece). Each portion was placed into a sterile bag (Whirl-Pak). Four bags for each sample were used; sample pieces were crushed thoroughly by a mortar, and then 20±0.5 ml of potassium phosphate buffer of different concentration and pH values (pH 4.5, 0.1M, pH 6, 0.1M, pH 8, 0.2M and pH 8, 0.1M) was added. Bags were labelled A to D. After well mixing, the suspension was allowed to settle for a minimum of 45 minutes. The supernatant fluid was collected and transferred to Eppendorf tube and used as an extract. The sample extracts were refrigerated if they would be held for more than two hours before use [9].
2.7. Assay Procedures
- Standard stainless steel bioassay cylinders were used to apply sample extracts on agar surface; the cylinders are 10 mm high, 6 mm diameter inside and 8 mm diameter outside. Five cylinders were placed on the surface of prepared plates as shown in (Figure 1), 200μl of sample extract were added into the cylinders.
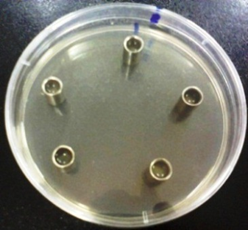 | Figure 1. Five bioassay cylinders on an inoculated agar plate surface |
|
2.8. Interpretation of Results
- Interpretation of results depends on four basic factors; the microorganism, media type, presence of penicillinase, and a residue level [9].
3. Results
- A sample is considered a positive sample (containing residues) when an extract inhibits the growth of bacteria on any plate with a zone of inhibition more than 8 mm in diameter.
3.1. Determination of Antibiotic Groups
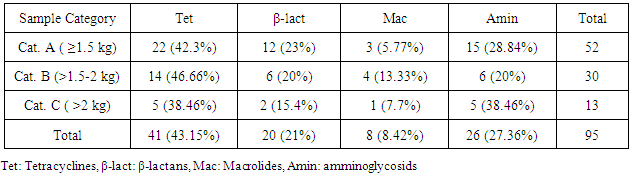
- The most detected antibiotic residues were tetracyclines 41/95 (43.15%) followed by aminoglycosides 26/95 (27.36%) then 20/95 (21%) and 8/95 (8.42%) for β-lactams and macrolides respectively as shown in (Table 4).
|
3.2. Detection of Antibiotics according to Samples Weight
- Residues were detected in 88 (24.1%) samples from all categories but the most detected residues were from category (A) (≤ 1.5 kg) 47 (53.41%), followed by 29 (32.95%) from category (B) (>1.5- 2 kg) and the least was category (C) (>2 kg) which were 12 (13.63%) as shown in (Figure 2).
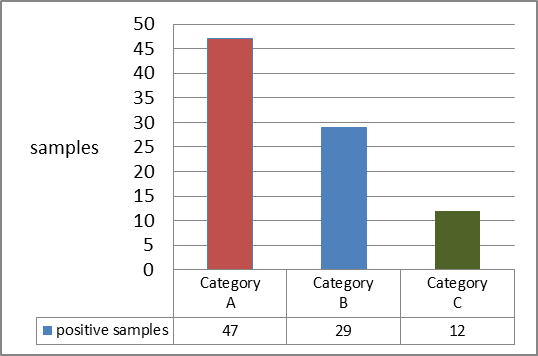 | Figure 2. Positive samples distribution according to carcasses weight |
3.3. Detection of Antibiotics According to Samples' Regions
- The highest percentage of antibiotic residues was detected in the middle region 34/121 (28.1%). Khanyounis and Rafah have the least percentage of residues that were detected in 24/110 (21.8%). Positive results distributed by regions are shown in (Table 5).
|
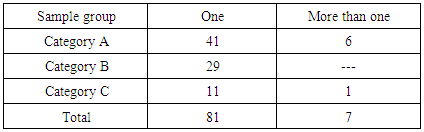
3.4. Detection of Multiple Antibiotics Residues
- Seven samples contained more than one residue. In category (A), six samples contained two different antibiotic residues (Table 6).
|
4. Discussion
- Antimicrobial residues in food of animal origin have received much attention in developed countries to ensure food safety, many countries have monitoring programs to avoid AMR in food of animal origin [10]. Currently, Palestine has no regulations regarding the use of antimicrobials or the maximum allowable antimicrobial concentrations in food. Additionally, there are no systems to monitor the presence of AMR in animal products in Palestine. Therefore, screening of food products from animal origin intended for human consumption for the presence of AMR is essential to ensure food safety.The 7-plate method [9] was used with a slight modifications; five plates were used instead of seven, two plates were excluded, one of them for streptomycin detection and the other for erythromycin confirmation.In this study a screening method was used to detect the presence of antibiotic residues therefore it could not be possible to proof their exceedance of the maximum residue limits. A confirmatory method like HPLC or GC is required to determine the type and the level of AMR in a sample [11].Similar results were recorded by other researchers from developing countries, 21%, 27%, 32.0% and 33% in Egypt, Sudan, Pakistan and Nigeria respectively [12-15]. But other researchers also from developing countries recorded high percentage of AMR 44%, 69.7%, 70.8%, 90%, in Iraq, Saudi Arabia, Sudan and Egypt respectively [16-19], these results could be logically found in developing countries due to the absence or weak monitoring and controlling programs of AMR in food of animal origin. The presence of AMR is definitely different in developed countries, in study in Bulgaria on 115 meat samples (pectoral muscles), only two samples (1.7%) were identified as AMR-positive [20]. In Belgium AMR in poultry meat were detected in 7.9% of samples [21].Investigations in countries where official regulations have been established demonstrate that regulations have been successful for controlling this issue. In United Kingdom in 2007, the surveillance for veterinary residues in broiler muscles revealed that one sample out of 1158 was had a sulfadiazine residues over MRLs [22].FSIS in USA in 2012 had screened 1474 samples from broilers and turkey for chemical compounds that include veterinary drugs, none of these samples was found to have any of the investigated chemical compounds [23]. In this study, tetracyclines group constituted 41(43.15%) of positive samples. This high result may be as a result of tetracyclines properties; since they are broad spectrum antimicrobials and can defend Rickettsia, Spirochetes, Chlamydia, and Mycoplasma infections [24]. In addition, they are produced by many drugs manufacturers and available in all veterinary clinics. It has been reported that tetracyclines are the most predominantly prescribed antimicrobials in Africa, and of all antimicrobial-associated residues they represent 41% of cases [25]. These results are comparable to other results from Arabic countries in Egypt [12, 19] and in Saudi Arabia [17, 26]. β-lactams residues were detected in (21%) of positive samples. In Gaza strip, the most commonly used β-lactams are cephalosporins, amoxicillin and ampicillin. Aminopenicillins have a short WP (1-2 days) in broiler chicken. Little information is available about WP of cephalosporins when used parenterally [27]. Macrolide group was detected in (8.42%). According to the questionnaire results the main macrolides used in Gaza strip are tylosin and erythromycin. Withdrawal period for tylosin and erythromycin is one day when used orally, and it was determined to be two days for erythromycin when used by injection [28]. As a result of the short WP, macrolides group was the least detected one.In this study broilers weight were used as a reflection of age since in standard conditions of closed broiler houses, broilers reach one and half kilos in about five weeks, and within the seventh week broilers may reach to two kilograms live body weight [29].The most antimicrobial residues were found in category (A), 47 samples out of 88 positive samples (53.4%) were from broiler carcasses weigh less or equivalent to 1.5 kilograms, this Chickens category needs highly specific environmental conditions e.g. temperature, ventilation and humidity, although theses conditions are important all over the period of breeding they are more crucial in the early stages of breeding, any fault in the management of a farm adversely effect chickens health specially to the respiratory system, therefore antimicrobials are used frequently, this condition may explains the high AR detection in category (A) samples.
5. Conclusions
- The study revealed that 24.1% of local broiler chickens retailed in Gaza strip contained one or more antibiotic agents belonging to one of the four investigated antibiotic groups (Tetracyclines, Aminoglycosides, β-Lactams and Macrolides). Antibiotic residues detected in higher frequency in lighter chickens than heavier ones. This high prevalence of antibiotic residues in broiler meat should be an alert for concerned authorities (Ministry of Agriculture, Ministry of Health and consumer protection organizations) to establish a national monitoring program of antimicrobial residues in foods.
ACKNOWLEDGMENTS
- We are extremely grateful to Palestinian Health Research Council for providing a research grant which helped in the accomplishment of this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML