-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2016; 6(4): 87-92
doi:10.5923/j.fph.20160604.01

Validation of a Method for Analysis of Avermectins Residues in Bovine Milk by HPLC-Fluorescence Detector
Nadia Regina Rodrigues1, Ana Paula Ferreira de Souza1, Paulo E. Rubbo2
1Analytical Chemistry Division, CPQBA, University of Campinas, Campinas-SP, Brazil
2Agroceres Multimix, Rio Claro-SP, Brazil
Correspondence to: Nadia Regina Rodrigues, Analytical Chemistry Division, CPQBA, University of Campinas, Campinas-SP, Brazil.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

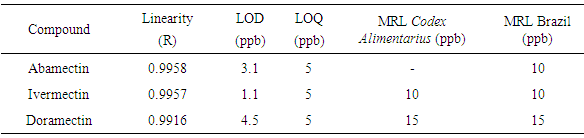
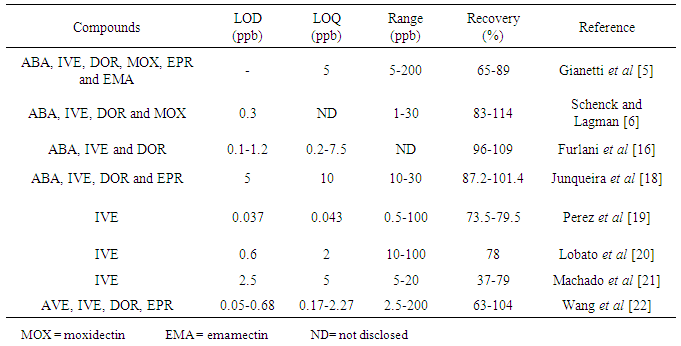
The presence of veterinary drug residues in bovine milk has raised the need to adapt their detection methodologies in milk for human consumption. The avermectins, antiparasitic class have been detected by liquid chromatography with fluorescence detection after derivatization reaction. The method validated in this work is fast and accurate for the determination of these residues in bovine milk according with limits establishment by CodexAlimentarius. The limit of quantitation (LOQ) for Abamectin, Doramectin and Ivermectin is 5 μg L-1 with a great recovery associated with a low coefficient of variation.
Keywords: Abamectin, Doramectin, Ivermectin, Lactation cows, Veterinary drugs
Cite this paper: Nadia Regina Rodrigues, Ana Paula Ferreira de Souza, Paulo E. Rubbo, Validation of a Method for Analysis of Avermectins Residues in Bovine Milk by HPLC-Fluorescence Detector, Food and Public Health, Vol. 6 No. 4, 2016, pp. 87-92. doi: 10.5923/j.fph.20160604.01.
Article Outline
1. Introduction
- Brazil is the fifth largest cow’s milk producer after the European Union, India, United States and China. Currently, the Brazilian milk production chain shows high growth potential, and the Ministry of Agriculture, Livestock and Food Supply (MAPA) projections to 2020 indicate that the production and consumption will be 37.75 and 33.27 billion liters, respectively [1, 2]. The use of veterinary drugs accompanies the growth of the Brazilian herd in order to ensure animal health and consequently increase livestock production. However, the indiscriminate use of these drugs during cow´s lactation can lead to the presence of residues in milk, even above the maximum residue levels (MRLs) [3, 4].Avermectins belong to the group of macrocyclic lactones and are produced by Streptomyces avermectilis. The main avermectins are Abamectin (ABA) which is a mixture of avermectin B1a and B1b, Ivermectin (IVE) is a mixture of two compounds 22,23-dihydroavermectin H2B1a and H2B1b and Doramectin (DOR) (Fig. 1) [5].
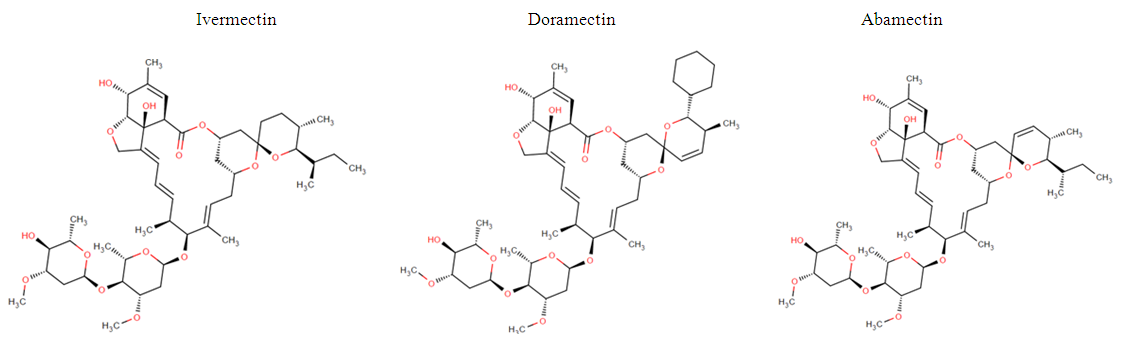 | Figure 1. Chemical structures of avermectins |
2. Materials and Methods
2.1. Standards and Reagents
- Standards of ABA, IVE and DOR were purchased from Sigma-Aldrich, with 99% of purity. The stock standard solutions were individually prepared in acetonitrile and stored at -20°C. The working standard solutions were prepared by diluting the stock solutions with methanol/ water/acetonitrile (40:5:55 v/v/v) in the concentration range of 2.5-25 µg L-1.Acetonitrile and Methanol HPLC grade were purchased from JT Baker (USA). Methylimidazole (MI) and trifluoroacetic acid (TFA) (Sigma-Aldrich, St. Louis, MO), triethylamine (Vetec, Brazil) and trifluoroacetic anhydride (TFAA) (Merck, Darmstadt, Germany) were of analytical grade. Water was obtained from a Milli-Q water system (Millipore, Bradford, MA, USA).
2.2. Sample Collection and Preparation
- The milk samples were purchased from a local organic farm and stored at -20°C. A total of 2.0 mL sample of milk was spiked by adding a standard solution to give fortification levels of 5, 10 and 15 µg L-1.Aliquots of 2 mL of milk were transferred to a 50 mL centrifuge tube and extracted with 5 mL of acetonitrile using a vortex mixer for 20 sec and then centrifuged for 10 min at 3000 g. The supernatant was transferred to a flask in which 13 mL of deionized water were added. A SPE Strata-X cartridge (Phenomenex, Torrance, CA) was conditioned with 3 mL acetonitrile and 3 mL acetonitrile: water (3:7 v/v). The extract was transferred into the cartridge and washed with 5 mL acetonitrile: water (3:7 v/v) and the avermectins were eluted into an ambar glass tube with 5 mL of acetonitrile. The resulting extract was evaporated under nitrogen flow at 58 °C.The dried extract was derivatizated with addition of 100 µL acetonitrile: MI (1:1), 150 µL acetonitrile: TFAA (2:1), 100 µL trimethylamine: acetonitrile (1:1) and 100 µL TFA: acetonitrile (1:1) using a micropipette. After, this solution was homogenizing in a vortex for 10 sec, filtered through a Millex HV filter (0.45 µm, Millipore) and analyzed by HPLC-FD [11].
2.3. HPLC-FD Analysis
- The analyses were performed on a HPLC-FD model Waters 2695 (Milford, MA, USA) equipped with a fluorescence detector. The compounds were separated under isocratic conditions into a C18 column (Symmetry, 75 x 4.6 mm, 3.5 µm) at 30°C and mobile phase methanol: water: acetonitrile (40:5:55 v/v/v). The flow rate was 1.0 mL min-1 and the excitation wavelength 365 nm and emission wavelength 475 nm. The total running time was 15 minutes and data acquisition was performed by Empower Waters software.
2.4. Method Validation
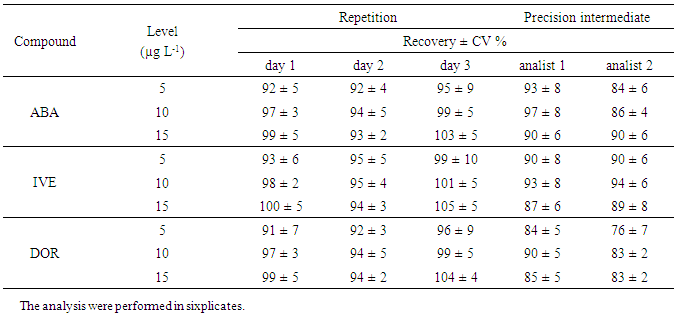
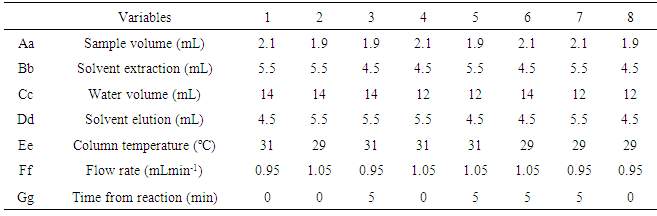
- The method validation was performed based on the criteria and recommendations of European Commission Decision 202/657/EC and also on good laboratory practice (GLP), supported in good scientific practice [12, 13]. The considered parameters were selectivity, linearity, precision, accuracy, limit of detection (LOD), limit of quantification (LOQ), matrix effect, stability and robustness.Selectivity was evaluated by analyzing 20 samples of blank milk. Linearity was determined in a range of 2.5-25 µg L-1 for ABA, IVE and DOR. Precision and accuracy were evaluated by spiking blank milk in three fortification levels for each analyte in the range of 5-15 µg L-1 with six replicates each one.Method precision was evaluated through the CV%. The analyses were performed on three different days by the same analyst and also by two different analysts at the same day. Limits of detection and quantification were estimated based on parameters of the calibration curve [14]. Robustness was performed using Youden´s test. Matrix effect was evaluated preparing six replicates in blank matrix extract and compared with the external calibration curve. This comparison was performed employing Student´s and Fisher’s test.
3. Results and Discussion
- In determining the avermectin residues in bovine milk, the derivatization procedure requires care and attention. The avermectins containing tetrahydrobenzofuran ring that were derivatized with acylating reagent in the presence of a strong base, were converted to a chromophore group resulting in a fluorescent aromatic derivative Fig. 2 [11, 15].
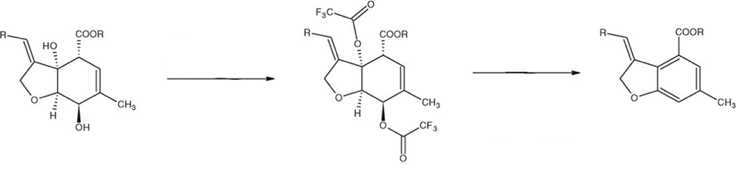 | Figure 2. Reaction: formation of a fluorescent chromophore [11] |
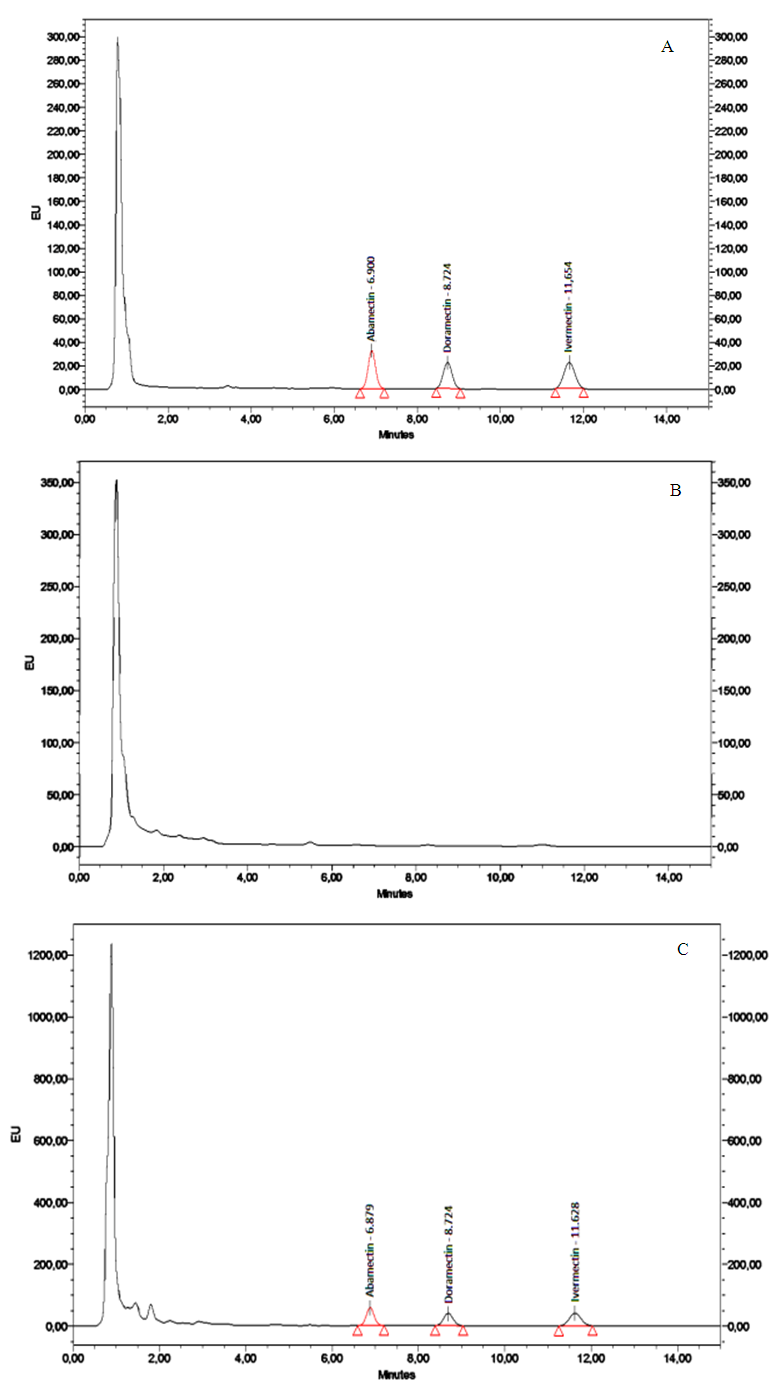 | Figure 3. HPLC-FD chromatograms of (a) standard solutions ABA, IVE and DOR at 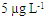 (b) blank milk sample (c) spike milk sample at (b) blank milk sample (c) spike milk sample at 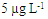 |
|
|
|
|
4. Conclusions
- This study demonstrates that methodology is applicable for the detection and quantification of Abamectin, Ivermectin and Doramectin residues in bovine milk. It is a low cost method, with good sensitivity and it uses a kind of detector accessible to any laboratory. The results showed that the use of small quantities of sample (2 mL) and organic solvent (5 mL) for extraction achieves the necessary accuracy and precision in compliance to the Brazilian legislation for monitoring of these residues. The use of small quantities of organic solvents also reduces the potentially toxic residues in the environment.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML