-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2016; 6(3): 75-85
doi:10.5923/j.fph.20160603.03

Effects of Dietary Fatty Acids on Human Health: Focus on Palm oil from Elaeis guineensis Jacq. and Useful Recommendations
Ntsomboh-Ntsefong Godswill1, 2, Likeng-Li-Ngue Benoit Constant1, 2, Bell Joseph Martin1, Tabi-Mbi Kingsley1, 2, Di-Maissou Jean Albert2, 3, Kenmogne Simo Thierry2, 3, Mana Ngangue Sastile2, 3, Ngalle-Bille Hermine1, Youmbi Emmanuel1, 4
1University of Yaounde 1, Department of Plant Biology, Yaounde, Cameroon
2Institute of Agricultural Research for Development, Specialized Centre for Oil Palm Research of La Dibamba, Douala, Cameroon
3University of Douala, Faculty of Medicine and Pharmaceutical Sciences, Douala, Cameroon
4Tissue Culture Laboratory, African Centre for Research on Banana and Plantain (CARBAP), Njombe, Cameroon
Correspondence to: Youmbi Emmanuel, University of Yaounde 1, Department of Plant Biology, Yaounde, Cameroon.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Most fatty acids or lipids are involved in the maintenance of membrane structures and in the survival of the plant at low temperature. These include conjugated or non-conjugated, trans fatty acids, short-, medium and long-chain saturated fatty acids (SFA), monounsaturated fatty acids (MUFA) (omega-7 and omega-9), and polyunsaturated fatty acids (PUFA) (omega-3 and omega-6). PUFA are indispensable for the proper functioning of the brain, the eyes, and the entire nervous system. However, uncontrolled intake of dietary lipids constitute a major health risk factor since they are associated with cardiovascular diseases (related to blood cholesterol levels), cancers, stroke, obesity, diabetes, autoimmune disorders and atherosclerosis, as well as ischemia-reperfusion injury and dementia risk. This paper presents the health effects of individual fatty acids and the interplay between them with a special focus on palm oil due to controversy on its dietary quality. The importance of some fatty acids is highlighted with strategies for improving dietary quality of oil through breeding and mutagenesis. Recommended optimal dietary fat profile is also presented with reference to standard norms or the position of some international health organisations. It is hoped that this paper will improve understanding on the effects and importance of dietary fatty acids especially of palm oil on human health.
Keywords: Cardiovascular disease, Breeding, Saturated fatty acids, Food and health, Mutagenesis, Palm oil
Cite this paper: Ntsomboh-Ntsefong Godswill, Likeng-Li-Ngue Benoit Constant, Bell Joseph Martin, Tabi-Mbi Kingsley, Di-Maissou Jean Albert, Kenmogne Simo Thierry, Mana Ngangue Sastile, Ngalle-Bille Hermine, Youmbi Emmanuel, Effects of Dietary Fatty Acids on Human Health: Focus on Palm oil from Elaeis guineensis Jacq. and Useful Recommendations, Food and Public Health, Vol. 6 No. 3, 2016, pp. 75-85. doi: 10.5923/j.fph.20160603.03.
Article Outline
1. Introduction
- Fruits and nuts may be considered as important components of a healthy diet. In general, they are nutrient-dense foods and provide protein, fat (mostly unsaturated fatty acids), dietary fiber, and many bioactive constituents such as vitamins, minerals, antioxidants [110] and other phytochemicals. Given such variety of bioactive compounds, extensive research has focused on potential health effects of higher nut and seed consumption on the development of heart disease and prostate cancer [47] [95] [22]. Dietary factors are associated with increasing levels of cardiovascular disease, certain types of cancers, stroke, obesity, non-insulin dependent diabetes mellitus, and atherosclerosis [53]. It is alleged that oil with high saturated fatty acid content is detrimental to health with regards to cardiovascular diseases and obesity. In this regard, though having unique beneficial properties [96], there is controversy on the health effects of palm oil consumption [32] [96] [1] because of its high content of saturated palmitic acid, which is known to increase low density lipoprotein cholesterol (LDL) in blood. Here we highlight the fact that research work on the oil palm should focus on selection for improved oil quality especially regarding its saturated fatty acids composition [118].However, it is worth noting that there is a complex interplay between the different series of dietary lipids, including conjugated or non-conjugated (animal or industrial) trans fatty acids, short-, medium and long-chain saturated fatty acids (SFA), various series (omega-7 and omega-9) of monounsaturated fatty acids, and the various series of PUFA, including omega-3 and omega-6 [20]. Fatty acids, the major constituents of oils (may include saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA) contribute to human physiology in different ways [68] [22]. Fatty acids are part of the composition of membranes of all plant tissues and of reserve lipids. Most fatty acids are involved in the maintenance of membrane structures and thus in the survival of the plant at low temperature [103]. Research has shown that PUFA are indispensable for the proper functioning of the brain, the eyes, and the entire nervous system. The PUFA are often classified into two families, n-3 and n-6, according to double bond positions. In spite of the important biological functions of both n-3 and n-6 groups, humans lack the enzymes required to form double bonds beyond the ω9 position. To overcome this limitation, the primary PUFA precursors, linoleic acid (LA, 18:2n-6) and α-linolenic acid (ALA, 18:3n-3), also called essential fatty acids (EFAs), must be obtained from a diet [112]. It has been evidenced that highly unsaturated n-3 fatty acids in moderate amounts do not increase LDL oxidizability when provided in the context of a diet rich in MUFA [56]. In general, all lipids and their interactions should be taken into account when analyzing the effect of dietary fat on cardiovascular diseases (CVD) complications and mortality [20]. Uncontrolled intake of fatty acids in their different forms can lead to health problems like increased risks of cardiovascular diseases (related to blood cholesterol levels) [25], cancer and autoimmune disorders [68] as well as ischemia-reperfusion injury and dementia risk [28] [76] [117]. The focus of this paper is to review the various effects of dietary fatty acids on these health issues with palm oil as a case study.
2. Health Effects of Some Individual Fatty Acids
2.1. Lauric and Myristic Acids
- Myristic acid (14:0), a dietary saturated fatty acid, is found in butter fat and in certain tropical oils like coconut oil and palm-kernel oil. Myristic acid is considered to be at least as hypercholesterolemic as palmitic acid [43], or even more harmful than palmitic or stearic acid [44] [20]. Lauric (C12:0) and myristic acids have a greater total cholesterol raising effect than palmitic acid. However, since myristic acid is present in much smaller amounts, its importance as a cholesterol-raising fatty acid for most diets is much less than for palmitic acid. Lauric and myristic acids raise total, LDL and HDL cholesterol and increases some hemostatic/thrombotic factors that promote thrombosis. These two fatty acids are abundant in tropical vegetable oils like coconut and palm kernel oils. Lauric acid alone decreases the total-to-HDL cholesterol ratio because of an increase in HDL cholesterol [69] [70].
2.2. Palmitic Acid
- Oils of oleaginous species contain relatively high contents of SFA notably palmitic (16:0) acid. Vegetable oils with low 16:0 and low stearic (18:0) acids are thus of great interest in the food industry [59]. The major saturated fatty acid in the diet is palmitic acid (16:0). Palmitic acid, a long-chain saturated fatty acid, also raises total, LDL and HDL cholesterol and increases some hemostatic/thrombotic factors that promote thrombosis. It is present in both animal and plant products and is the predominant saturated fatty acid in most meat and dairy fats, but a substantial fraction of palmitic acid in the diet comes from plant products. A major reduction in palmitic acid intake could be achieved by specifically curtailing intakes of animal fats. Palmitic acid makes up 45% of fatty acids in palm oil, 25% in cottonseed oil, and lesser amounts in other plant oils. There is evidence from metabolic and epidemiologic studies [51] [45] [64] [42] [50] that palmitic acid raises the serum total cholesterol concentration, compared with unsaturated fatty acids or carbohydrate [43]. A high blood cholesterol level is also associated with an increased risk of heart disease. In fact, cholesterol is found in the blood in two main forms, in complexes with either high-density lipoprotein (HDL-C) or low-density lipoprotein (LDLC). It is high levels of the latter form that are associated with heart disease risk, with HDL-C being neutral or perhaps beneficial [23]. Thus, measurements of LDL-C levels, or of the LDL-C: HDL-C ratio, are likely to be more useful than total cholesterol [20]. A contrary view point according to which epidemiological data do not support a link between dietary cholesterol and CVD is that dietary cholesterol content does not significantly influence plasma cholesterol values [60]. However, it has been reported that the increase in total cholesterol concentrations induced by palmitic acid occurs predominantly in the low-density lipoprotein (LDL) fraction. Slight increases also may occur in high-density lipoproteins (HDLs) and very-low-density lipoproteins (VLDLs). The mechanism whereby palmitic acid raises LDL-cholestenol concentrations is not fully understood, but there is growing evidence that it acts to suppress the expression of LDL receptors [105] [80]. Some studies investigating the effects of palmitic acid in humans [64] [42] [13] showed that palmitic acid raises LDL-cholesterol concentrations relative to unsaturated fatty acids (or carbohydrate). These studies leave little doubt that dietary palmitic acid is hypercholestenolemic. Furthermore, this fatty acid accounts for most of the cholesterol-raising action of most diets rich in SFA [43].However, palmitic acid is considered to be harmful only if the level of linoleic acid in the diet is very low. Palmitic acid may also have undesirable effects if the diet is high in cholesterol [44] [20].
2.3. Stearic Acid
- Stearic acid (C18:0), a long-chain saturated fatty acid, in contrast with other SFA apparently does not raise serum cholesterol concentrations. Investigations that specifically compared stearic acid with other fatty acids in human studies have confirmed that this fatty acid does not raise low-density-lipoprotein cholesterol and as such is not hypercholesterolemic [43]. Animal fats contain predominantly palmitic and stearic acids. Common food sources of stearic acid include most fats and oils, cocoa butter and fully hydrogenated vegetable oils. Stearic acid has a neutral effect on total, LDL, or HDL cholesterol [57] [83] [70] [84].
2.4. Oleic Acid
- Evidence indicates that most SFA raise serum cholesterol concentrations relative to monounsaturated oleic acid [51] [45] [42] [13]. Oleic acid (18:1) is known to be neutral in its effects on cholesterol concentrations [43]. It is generally considered to be a neutral fatty acid, neither raising nor lowering cholesterol concentrations. The concept of the neutrality of oleic acid is used as a baseline with which to judge the responses of other nutrients (both fatty acids and carbohydrates) [25]. However, it has been noted that 18:1 is at least as effective as polyunsaturates in lowering total cholesterol and improving the LDLC: HDL-C ratio [90]. In some studies, food sources of oleic acid were associated with a low risk of breast cancer [63] [58] but meta-analysis found that tissue oleic acid levels were positively associated with breast cancer [89] [85].The level of oleic acid in blood and tissue is more dependent on endogenous metabolism than on dietary intake [25]. The main enzymatic system regulating oleic acid level is the delta-9 desaturase (also called stearoyl-coenzyme A desaturase) and its activity depends on dietary (carbohydrate intake), hormonal (insulin) and lifestyle (physical exercise) factors. Thus, blood concentration of oleic acid is not a surrogate of the consumption of oleic acid but rather a biomarker of lifestyle associated with insulin resistance, which is by itself positively associated with the risk of breast cancer [25]. It is important to increase oleic acid (18:1) content since oil with high content of 18:1 is of great interest. It presents hypocholesterolemic properties that contribute in the prevention of heart diseases [59]. Such oil is relatively less sensitive to oxidation at high temperature [106] and as such is appropriate for frying [59]. Several studies to characterize the genetic determinism of the high 18:1 contents phenotype have led to contradictory results [84]. However, mutants presenting high 18:1 contents in their seeds have been obtained on many oil producing species like soybean, sunflower, rapeseed and groundnuts with the exception of olive that is naturally rich in oleic acid [59]. However, dietary oleic acid is not necessarily a marker of olive oil consumption as it is also one of the main fatty acids of meat [25]. Chemical mutagenesis was used to obtain rapeseed and Brassica rapa mutants with high 18:1 contents and low contents of poly unsaturated fatty acids [3] [93] [59]. A genetic study of the mutants revealed that the high 18:1 content phenotype is controlled by the same locus and that the two mutations affect alleles at this same locus [59]. RFLP gene markers linked to this locus were identified thereby revealing the fact that the locus affected by mutation leading to high 18:1 content corresponds to a gene of the endoplasmic reticulum [93]. Other studies on soybean and groundnuts revealed similar results [86] [87] [108]. Depending on the normal genotype used, the high 18:1 content phenotype of seeds can be under the control of one locus or of two loci. A particular population of sunflower obtained by chemical mutagenesis presented high 18:1 content [104] and new varieties with high 18:1 contents were obtained from the latter populations and have been cultivated for over 30 years. It has been found that high oleic mutant lines of sunflower (Helianthus annuus L.) are controlled by a certain number of genes. The existence of a single dominant gene, Ol, has been reported by [35], while a major gene, Ol, and a gene modifier, Ml have also been discovered by Miller and collaborators (1987) cit. [103]. Three complementary genes, Ol1, Ol2 and O3, were also reported by [34], whereas [27] found the Ol gene with incomplete penetrance determined by genotypic epistatic factors of reversion. Five genes (Ol1, Ol2, Ol3, Ol4 and Ol5) were also presented by [115], while [59] found a high oleic locus, oleHOS, and a suppressor locus, Sup. Regarding the intake of oleic acid, it is critical to differentiate the food sources, since the health effects of 18:1 obtained from meat or from olive oil are different. The best approach is probably the traditional Mediterranean diet model [24].
2.5. n-3 and n-6 Fatty Acids
- It has been reported that the type of fatty acids in the maternal diet affects the fatty acid composition of breast milk [38] Consumption of polyunsaturated vegetable oils raises the milk content of linoleic and linolenic acids [36] [91]. Linoleic acid (18:2n-6) and linolenic acid (18:3n-3) are essential fatty acids. The n-6 Linoleic acid (18:2) is a long chain fatty acid whose intake helps to prevent omega-6 deficiency. In fact, its importance relates to its ratio in the diet (of the n-6 fatty acids) to the n-3 fatty acids. An imbalance in this ratio can accentuate the n-3 fatty acid deficiency state. Interest in the n-3 fatty acids began over forty years ago from studies in the Greenland Eskimo by Dyerberg and Bang. Despite a high fat diet, these Eskimos had a low prevalence of coronary heart disease because their fat contained high proportions of n-3 fatty acids. From thence, much research has been undertaken about n-3 fatty acids [19]. The n-3 fatty acids are very important in human nutrition. They are significant structural components of the phospholipid membranes of tissues throughout the body and are especially rich in the retina, brain [52], and spermatozoa in which docosahexaenoic acid (22:6 n-3 or DHA) constitutes up to 36.4% of total fatty acids [62]. These are all structures where membrane fluidity is essential for proper functioning. In the retina, n-3 fatty acids are especially important. n-3 fatty acid deficiency has resulted in decreased vision and abnormalities of the electroretinogram [19]. Moreover, fish and omega-3 (n-3) polyunsaturated fatty acid (PUFA) intake may reduce dementia risk [28]. In fact, Dementia, also known as senility, is a broad category of brain diseases that cause a long term and often gradual decrease in the ability to think and remember that is great enough to affect a person's daily functioning [117].Both n-3 and n-6 fatty acids are essential fatty acids. Only linoleic and linolenic acids are found in cow’s milk. The essential fatty acid status of cow’s milk is however questionable even though the ratio of n-6 linoleic acid to the n-3 linolenic acid is appropriate and is in the 2 to 1 range. Modified cow’s milk has 2% of n-6 fatty acids and 1% of n-3 fatty acids in terms of percent of total calories. A typical corn oil-coconut oil formula has high amount of the n-6 linoleic acid and very little of the n-3 linolenic acid. In the USA such formulas were changed in the 1980’s and soybean oil which has a very good ratio of linoleic to linolenic acid, seven to one, was introduced [19]. The n-3 fatty acids help to modulate and prevent human diseases, particularly coronary heart disease. Their effects on the brain later in life to prevent certain disorders like Alzheimer’s disease need to be confirmed by further studies. The evidence is now very strong that n-3 fatty acids are essential for human development in the foetus and infant. The antiarrythmic effect of n-3 fatty acids is a discovery that has great relevance to the prevention of sudden death from ventricular fibrillation in patients with coronary heart disease [19].Apart from the useful health effects of linolenic acid (18:3), it contains three double bonds and is thus very sensitive to oxidation which results in an odour and an undesirable taste of oil. So reduction of 18:3 content will improve on the quality of any dietary oil. To this effect, mutagenesis has been successfully employed on rapeseed and soybean to produce mutants with low 18:3 contents in grains. In soybean, a cross of two different mutants with low 18:3 contents obtained by mutagenic treatment yielded progeny with lower 18:3 contents than those of the two parents [33]. However, the genetic control of the phenotype low 18:3 content in soybean seeds seems to be relatively complex, depending on at least 2 loci with additive effects on the character [59].
3. Saturated, Unsaturated and trans Fat Contents in Vegetable Oils
- Saturated fatty acids (SFA) have specific fluidity characteristics. They serve in the composition of several dietary and non-dietary fats like margarine. Vegetable oils with high SFA contents are of great industrial interest since they limit the steps involving hydrogenation to obtain a pure source of SFA. For this reason, it could be interesting to develop oil crop genotypes that produce oil with increased SFA content. Nonetheless, high amounts of SFA at the level of all tissues of the entire plant is lethal and as such, an increase of SFA contents has to be specific in seeds and fruits. Mutagenesis could be geared at increasing SFA (SFA) content. In this wise, success has been achieved on Brassica rapa and rapeseed [55] where transformation led to the selection of transformed plants with 18 :0 contents in grains of each of the two species up to 25 times higher than for untransformed plants. However, this transformation for Brassica rapa is correlated with great reduction in oil content of grains as well as a very low germination potential [59].It appears that trans acids in the diet have physiological effects similar to, or more harmful than SFA. The view that trans acids should be avoided is gaining strength [78]. With increasing concern over the harmful effects of trans fatty acids in the diet, a demand for ‘trans-free’ margarine has developed. This may represent a significant market for palm oil since trans-free products can be produced using palm and palm kernel oils as sources of solid fat [8], interesterified with liquid oils. Several recent studies have shown that trans acids tend to increase total cholesterol, and to reduce HDL-C and increase LDL-C levels [20].Naturally, all unsaturated fatty acids (FA) in the vegetable oils are in the cis-form; while, a large proportion of unsaturated FA isomerize to their trans FA counterparts during industrial hydrogenation of vegetable oils. Thus, dietary fats made with fully and/or partially hydrogenated oils such as margarines contain trans FA [2] [9]. Highly unsaturated vegetable oils are less suitable for many food applications [80] [92]. However, hydrogenated fats and oils prevent rancidity and are used in foods to improve texture and stability for a longer shelf life because trans FA have higher melting points and greater stability than their cis isomers [116] [29]. Bakery products made with such hydrogenated fats and oils such as biscuits, cakes, cookies, crackers and breads also contain trans FA [31].
4. Norms and Recommended Dietary Fat Profile
4.1. Recommendations
- Since SFA increase the risks of cardiovascular diseases, cancer and autoimmune disorders, oils are of more nutritional value if they have proportionally more unsaturated than SFA [68]. Reducing saturated fatty acid (SFA) intakes is still at the heart of dietary recommendations to reduce CVD, mainly because of its effect on blood cholesterol [25]. The lipoproteins are not the only components of blood affected by the fats in the diet, and there are many interactions among different dietary components. However, the optimal dietary fat profile includes a low intake of both saturated and omega-6 fatty acids and a moderate intake of omega-3 fatty acids. The high average intake of omega-6 PUFA in Western countries may explain the persistently high rate of CVD complications and the increased incidence of certain cancers, including breast cancer. Some studies have found a positive association between omega-6 and breast cancer risk, contrary to omega-3 fatty acids which do have anticancer properties. Therefore, high omega-3 to omega-6 ratio may be the optimal strategy to decrease breast cancer risk. A moderate intake of plant and marine omega-3 in the context of the traditional Mediterranean diet (low in saturated and omega-6 fatty acids but high in plant monounsaturated fat) appears to be the best approach to reduce the risk of both cardiovascular diseases and cancers, in particular breast cancer [25].
|
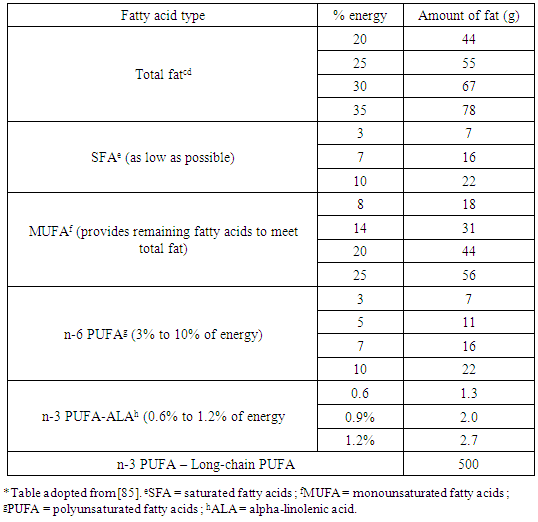
4.2. International Norms
- No group of natural fatty acids is ‘better’ than the other. The American Heart Foundation recommends that the fat component of the diet should consist of equal proportions of saturated, monounsaturated and polyunsaturated fatty acids [20]. Many health organizations have recommended that intakes of SFA be decreased in an effort to reduce serum cholesterol concentrations and thereby decrease the risk for coronary heart disease (CHD). Since various SFA have differing effects on serum cholesterol concentrations, it has been proposed that the general recommendation to reduce intakes of SFA as a class be reconsidered. It is the position of the Academy of Nutrition and Dietetics that dietary fat for the healthy adult population should provide 20% to 35% of energy, with an increased consumption of n-3 polyunsaturated fatty acids and limited intake of saturated and trans fats. The Academy recommends a food-based approach through a diet that includes regular consumption of fatty fish, nuts and seeds, lean meats and poultry, low-fat dairy products, vegetables, fruits, whole grains, and legumes [41]. The same position as above is upheld by the American Disease Association (ADA) and Dietitians of Canada (DC). ADA and DC recommend a food-based approach for achieving the fatty acid recommendations, notably a dietary pattern high in fruits and vegetables, whole grains, legumes, nuts and seeds, lean protein, fish, and use of non hydrogenated margarines and oils [85].Therefore reduction or replacement of dietary saturated fat with polyunsaturated fat is still the main dietary strategy to prevent cardiovascular diseases. The optimal dietary fat pattern to reduce the risk of both cardiovascular disease (CVD) and most cancers should include a low intake of saturated fatty acids (SFA) and omega-6 PUFAs. Small amounts of the essential linoleic acid (easy to find in most Western foods) are sufficient to prevent omega-6 deficiency. The intake of omega-3 PUFA, from plant and marine sources, should be moderate [25].
5. Improving Fatty Acids Profile: Focus on Palm Oil
5.1. Fatty Acid Profile of Palm Oil
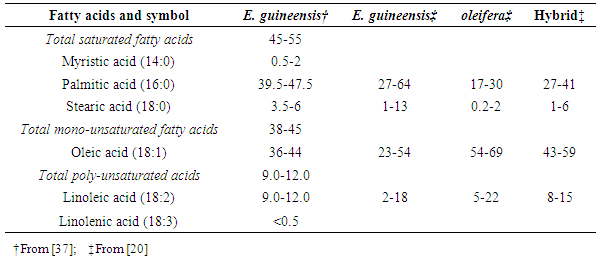
- For decades now, there has been controversy on the health effects of palm oil consumption [32] [96] [1] because of its high content of saturated palmitic acid. In fact, palm oil has a mixture of polyunsaturated, monounsaturated and saturated fatty acids with palmitic acid (16:0) (40%), stearic acid (18:0) (5%), oleic acid (18:1) (44%) and linoleic acid (18:2) (10%) constituting the four main fatty acids [17] [15]. Palmitic acid, the principal constituent of refined palm oil increases low density lipoprotein cholesterol (LDL) in blood. Dietary quality norms recommend an average content of 39-47.5% of 16:0 (saturated palmitic acid) and 36-44% of 18:1 (mono-unsaturated oleic acid) for palm oil [107]. Moreover, health agencies in Western countries have been warning about the health hazards of excessive intake of dietary fats, especially of those rich in saturated fats [16]. World Health Organization (WHO) forecasts that the incidence of these two health problems is to rise strongly during the next decades in Africa! There is therefore an utmost need for research work on the oil palm to focus on selection for improved oil quality especially regarding its saturated fatty acids composition [118]. Breeding and genetic improvement of the fatty acid profile of palm oil from Elaeis guineensis Jacq. can be achieved by introgression of the trait from Elaeis oleifera, its close relative. The ranges of fatty acids content in palm oil from Elaeis guineensis, Elaeis oleifera and E. guineensis x E. oleifera hybrid are presented in Table 2.
|
5.2. Minor Constituents of Palm Oil
- Palm oil contains a variety of minor components all at concentrations of less than 1000 ppm. These include carotenoids, tocopherols, chlorophyll, sterols, phosphatides and alcohols [20]. The most important of these are α- and β-carotene, which are precursors of vitamin A, and tocopherols and tocotrienols (vitamin E). Of the carotenes, β-carotene has the strongest provitamin A activity. Carotenoids are important in human health [79]. Vitamin A deficiency can lead to blindness, and the use of red palm oil is a good prophylactic of this condition. Tocotrienols have protective effects against heart disease and cancer [90]. The carotenes which give the oil its colour are destroyed during conventional refining, hydrogenation and fractionation, and about 30% of the tocopherols are also lost [39]. The importance of the nutritional value of these components has led to the development of methods of extracting them from palm oil. Ong et al. [81] described the extraction of vitamin E from palm fatty acid distillate (the FFA removed in physical refining), which contains 0.4 - 0.8% of tocopherols and tocotrienols. Methods of concentrating or extracting carotenes have been described by [82] [61] [4].There is some evidence that palm oil may inhibit the formation and reduce the growth rate of tumours, but these effects appear to be independent of its fatty acid composition, and may be attributable to some of the minor components in the oil [20].
5.3. Dietary Oil Quality Improvement through Mutagenesis
- Various mutagenic and transgenic programs have successfully been undertaken on oleaginous species like soybean, sunflower and rapeseed to select genotypes with low SFA (16:0 and 18:0) contents in grains or fruits. Earlier on, due to the complexity and the high number of enzymes involved in the synthesis of palmitic acid (16:0), there was little knowledge about the gene(s) affected by such mutations [54]. Most studies have revealed the complexity of the control of low contents of 16:0 or 18:0 in grains of different oleaginous species [59]. Chemical mutagenesis which has been successful with several plants can serve as an approach to improve on palm oil dietary quality.Research work on biotechnology of oil palm has been underway for some time through a multidisciplinary approach involving biochemical studies, gene and promoter isolation, transformation vector construction and genetic transformation to produce targeted products with a main target to increase oleic acid in the oil palm mesocarp. Other targets are stearic acid, palmitoleic acid, ricinoleic acid, lycopene (carotenoid) and biodegradable plastics [84]. Studies have successfully led to the isolation of useful oil palm genes and characterization of important promoters which have been used to produce a large number of transformation constructs for various targeted products. The regeneration of transgenic oil palm harbouring the useful genes is in progress [83] [84]. Traditional breeding effectively results in oil palm improvement but is however impeded by the long selection cycle of about 10 to 12 years and the enormous resources involved in land, labour and field management of the breeding programs. The time and resources could be greatly reduced through the use of modern approaches like biotechnology [97]. It is therefore necessary that breeders adopt innovative technologies such as modern genetics and genetic engineering [94], which can increase the efficiency of selection with more precision [18] [109]. Such innovative technologies will assure rapid and precise selection for the traits of interest. These include high throughput genomics technologies like next generation sequencing and high throughput genotyping which help in understanding the functions and regulation of genes in crop plants [114]. These techniques have been used in oil palm to understand oil formation [14] [111] [30]. Thanks to them, oil palm genome has also been sequenced [88] [98]. The use of genetic maps [67] [75] [10] [100] [11] and molecular markers [66] like Quantitative Trait Loci (QTL) [11] [72] have helped and are in progress to genetically improve the oil palm [48] [6] [46] [65] [66] [11]. QTLs for fatty acid composition of palm oil from intraspecific cross of E. guineensis and from an interspecific cross with E. oleifera [72] have been described. These revealed that several genes with additive effects impact fatty acid composition of palm oil and corroborate with the number of candidate enzymes involved in late fatty acid synthesis and edition process [5]. Moreover, genetics and genomics studies involving the fatty acid composition in the Elaeis genus have shed some light about genes, enzymes and regulatory mechanisms for fatty acid composition traits [101] [111] [14] [73]. The preceding reveals that the control of fatty acids contents of oil seeds is complicated and depends on complex genetic and environmental factors. Modification of fatty acids content through transgenesis is promising though faced with many constraints. Hence, a natural selection scheme based on analysis of fatty acids contents of different oil palm populations and detection of elite palm trees which naturally produce good dietary quality oil is seemingly the best approach at present.
6. Conclusions
- Cardiovascular diseases and cancers are leading causes of morbidity and mortality [25]. In a study on fat consumption and mortality due to cardiovascular disease, the analysis of consumption patterns of meat, vegetable and dairy fats in countries with high and low cardiovascular mortality did not allow categorical statements such as observed in the past [113], even though countries with low mortality showed higher availability of vegetable fat and countries with high mortality exhibited higher availability of animal fat for consumption [71]. However, all lipids and their interactions [102] should be taken into account when analysing the effect of dietary fat on cardiovascular diseases (CVD) complications and mortality [20]. There is now a consensus about recommending the Mediterranean diet pattern for the prevention of coronary heart disease (CHD) and cancer. The most important aspect of this treatment decision, in contrast with the pharmacologic prevention of CHD (including cholesterol lowering), is that the Mediterranean diet has a striking effect on survival [24]. The oil palm has long been denigrated for its oil quality related to CVD in consumers [16] [20]) with the assumption that because the oil contains 50% saturated fatty acids, it would behave like other saturated fats [16]. Nonetheless, no dietary pattern has been so extensively studied, and no other has been shown to provide so many benefits without any adverse effects [25]. It is however important to consider improving fatty acid composition of dietary vegetable oils to enhance health and safety. In this wise, mutagenesis and transgenesis have been used on some oleaginous species to develop new varieties with modified fatty acids contents. Also, the mode of inheritance of fatty acid content in mutant lines with high (low) levels of palmitic and stearic acids has been discovered [103]. The useful information provided by genetic maps, QTLs [99] and marker type Single Nucleotide Polymorphisms (SNPs) involved in fatty acid composition [73] are very useful since they could facilitate the implementation of classical breeding strategies aimed at modifying the fatty acid composition [72] and free fatty acid content [74] of palm oil from E. guineensis and dietary oils from other vegetable oil crops.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML