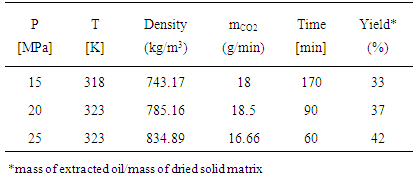
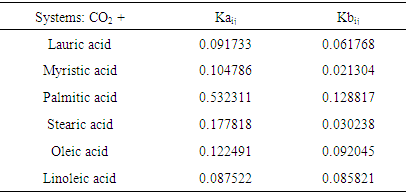
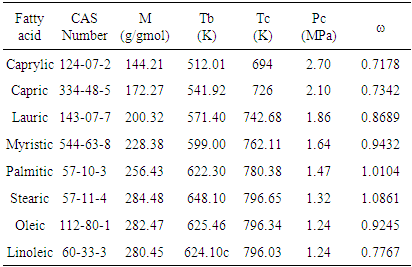
| [1] | J. Baltes, Gewinnung und Verarbeitung von Nahrungsfetten. Verlag Paul Parey, Berlin- Hamburg, 1975. |
| [2] | C. W. Chen, C. L. Chong, H. M. Ghazali, O. M. Lai, “Interpretation of triacylglycerol profiles of palm oil, palm kernel oil and their binary blends”. Food Chem 100(1): 178–191, 2007. |
| [3] | T. P. Pantzaris, M. J. Ahmad, “Properties and utilization of palm kernel oil”. Palm Oil Dev 35:11–23, 2001. |
| [4] | M. Herrero, M. C. Puyana, J. A. Mendiola, E. Ibañez, “Compressed fluids for the extraction of bioactive compounds”. Trends in Analytical Chemistry, v. 43, p. 67-83, 2013. |
| [5] | J. Martinez and A. C. Aguiar, “Extraction of triacylglycerols and fatty acids using supercritical fluids – Review”. Current Analytical Chemistry, 10, 67-77, 2014. |
| [6] | F. Sahena, I. S. M. Zaidul, S. Jinap, A. A. Karim, K. A. Abbas, N. A. N. Norulaini, A. K. M. Omar, “Application of supercritical CO2 in lipid extraction – e review”. J. of Food Engineering, 95: 240-253, 2009. |
| [7] | M. Herrero, J. A. Mendiola, A. Cifuentes, E. Ibañez, “Supercritical fluid extraction: Recent advances and applications. J. of Chromatography A, 1217:2495-2511, 2010. |
| [8] | M. N. Moraes, G. L. Zabot, M. A. A. Meireles, “Applications of supercritical fluids in Latin America: Past, present and future trends”. Food and Public Health, 4(3): 162-179, 2014. |
| [9] | R. Vardanega, J. M. Prado, M. A. A. Meireles, “Adding value to agri-food residues by means of supercritical technology”. The Journal of Supercritical Fluids, v. 96, p. 217-227, 2015. |
| [10] | M. Kalani, R. Yunus, “Application of supercritical antisolvent method in drug encapsulation: a review”. Int. J. of Nanomedicine, 6:1429-1442, 2011. |
| [11] | D. T. Santos, J. Q. Albarelli, M. M. Beppu, M. A. A. Meireles, “Stabilization of anthocyanin extract from jabuticaba skins by encapsulation using supercritical CO2 as solvent”. Food Research International, v. 50, p. 617-624, 2013. |
| [12] | E. K. Silva and M. A. A. Meireles, “Encapsulation of food compounds using supercritical technologies: Applications of supercritical carbon dioxide as an antisolvent”. Food and Public Health, 4(5): 247-258, 2014. |
| [13] | L. Vásquez, A. M. Benavides-Hurtado, G. Reglero, T. Fornari, E. Ibánez, F. J. Senorans, “Deacidification of olive oil by countercurrent supercritical carbon dioxide extraction: Experimental and thermodynamic modeling”. Journal of Food Engineering, v. 90, p. 463-470, 2009. |
| [14] | L. Vásquez, C. F. Torres, T. Fornari, F. J. Senorans, G. Reglero, “Recovery of squalene from vegetable oil sources using countercurrent supercritical carbon dioxide extraction”. Journal of Supercritical Fluids, v. 40, p. 59-66, 2007. |
| [15] | T. Fornari, L. Vásquez, C. F. Torres, E. Ibánez, F. J. Señoráns, G. Reglero, “Countercurrent supercritical fluid extraction of different lipid-type materials: Experimental and thermodynamic modeling”. Journal of Supercritical Fluids, v. 45, p. 206-212, 2008. |
| [16] | K. Gast, N. T. Machado, G. Brunner, “Countercurrent extraction of vitamines from crude palm, In State of the Art Book on Supercritical Fluids”, p. 267-280, ANIA Press, 2004. |
| [17] | G. Brunner and N. T. Machado, “Process design methodology for fractionation of fatty acids from palm fatty acid distillates in countercurrent packed columns with supercritical CO2”. The Journal of Supercritical Fluids, 66:96-110, 2012. |
| [18] | R. Dohrn, J. M. S. Fonseca, S. Peper, “Experimental methods for phase equilibria at high pressures”. Annu. Rev. Chem. Biomol. Eng. 3:343-67, 2012. |
| [19] | H. Sovová, Z. Marie, V. Miroslav, S. Karel, “Solubility of two vegetable oils in supercritical carbon dioxide”. The Journal of Supercrit. Fluids, 20, 15-28, 2001. |
| [20] | R. N. Carvalho Jr., L. S. Moura, P. T. V. Rosa, M. A. A. Meireles, “Supercritical fluid extraction from Rosemary (Rosmarinus officinalis): Kinetic data, extract’s global yield, composition, and antioxidant activity”. The Journal of Supercritical Fluids, 35, 197-204, 2005. |
| [21] | C. S. G. Kitzberger, R. H. Lomonaco, E. M. Z. Michielin, L. Danielski, J. Correia, S. R. S. Ferreira, “Supercritical fluid extraction of shiitake oil: Curve modeling and extract composition “. Journal of Food Engineering, 90, 35-43, 2009. |
| [22] | S. G. Ozkal, M. E. Yener, L. Bayindirli, “The solubility of apricot kernel oil in supercritical carbon dioxide”. International Journal of Food Science & Technology, 41, 399-404, 2005. |
| [23] | K. Tomita, S. Machmudah, A. T. Quitain, M. Sasaki, R. Fukuzato, M. Goto, “Extraction and solubility evaluation of functional seed oil in supercritical carbon dioxide”. The Journal of Supercritical Fluids 79, 109-113, 2013. |
| [24] | M. N. Hassan, N.N. Ab. Rahman, M.H. Ibrahim, A. K. M. Omar, “Simple fractionation through the supercritical carbon dioxide extraction of palm kernel oil”. Separation and Purification Technology, 19, 113-120, 2000. |
| [25] | N.A. N. Norulaini, I. S. Md. Zaidul, O. Anuar, A. K. M. Omar, “Supercritical enhancement for separation of lauric acid and oleic acid in palm kernel oil (PKO)”. Separation and Purification Technology, 35, 55-60, 2004. |
| [26] | J. E. Rodrigues, M. E. Araújo, F. F. M. Azevedo, N. T. Machado, “Phase equilibrium measurements of Brazil nut (Bertholletia excelsa) oil in supercritical carbon dioxide”. The Journal of Supercritical Fluids, 34, 223-229, 2005. |
| [27] | M. E. Araújo, N. T. Machado, M. A. A. Meireles, “Modeling the phase equilibrium of soybean oil deodorizer distillates + supercritical carbon dioxide using the Peng-Robinson EOS”. Industrial & Engineering Chemistry Research, 40:1239-1243, 2001. |
| [28] | T. Fornari, P. Luna, R. P. Stateva, “The vdW EoS hundred years later, yet younger than before. Application to the phase equilibria modeling of food-type systems for a green technology”. J. of Supercritical Fluids, 55, 579-593, 2010. |
| [29] | S-A. Hong, J-D. Kim, J. Kim, J. W. Kang, I-J. Kang, “Phase equilibria of palm oil, palm kernel oil, and oleic acid +supercritical carbon dioxide and modeling using Peng-Robinson EoS”. Journal of Industrial and Engineering Chemistry, 16, 859-865, 2010. |
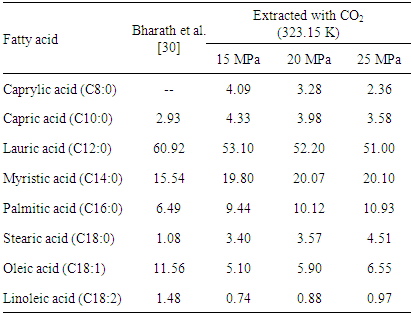
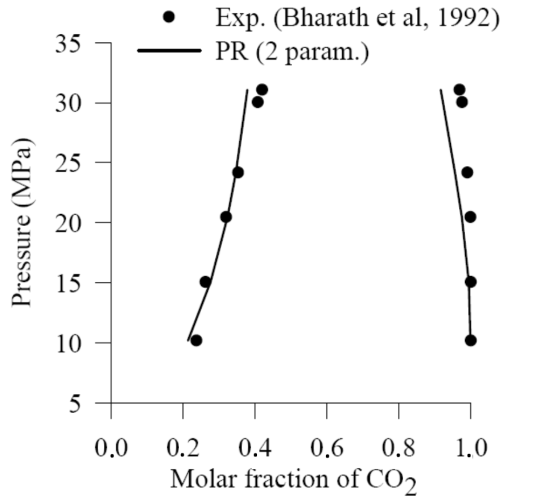
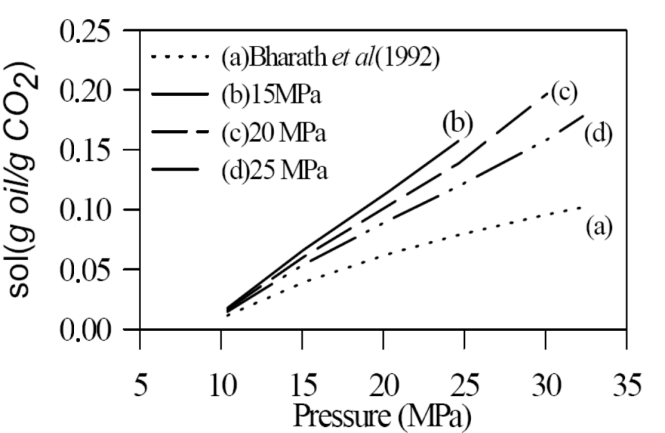
| [30] | R. Bharath, H. Inomata, T. Adschiri, K. Arai, “Phase Equilibrium study for the separation and fractionation of fatty oil components using supercritical carbon dioxide”. Fluid Phase Equilibria, 81, 307-320, 1992. |
| [31] | M. E. Araújo and M. A. A. Meireles, “Improving phase equilibrium calculation with the Peng-Robinson EoS for fats and oils related compounds/supercritical CO2 systems”. Fluid Phase Equilibria, 169, 49-64, 2000. |
| [32] | J. A. Nelder and R. Mead, “A simplex method for the function minimization”. Computer Journal, v. 7, p. 308-313, 1965. |
| [33] | B. Buczek and D. Geldart, “Determination of the density of porous particles using very fine dense powders”. Powder Technol., 45:173, 1986. |
| [34] | “American Society of Agricultural Engineers. Method of determining and expressing fines of feed materials by sieving”. American Society of Agricultural Engineers Standards, 1993. |
| [35] | I. S. M. Zaidul, N. A. N. Norulaini, A. K. M. Omar, R. L. Smith Jr., “Supercritical carbon dioxide (SC-CO2) extraction of palm kernel oil from palm kernel”. Journal of Food Engineering, 79, 1007-1014, 2007. |
| [36] | K-W. Quirin. Fette Seifen Anst., 84, 460, 1982. |
| [37] | E. Stahl, K-W. Quirin, D. Gerard. Fette, Seifen, Anstrichn, 85:458, 1983. |
| [38] | E. Stahl, K-W. Quirin, D. Gerard, “Dense Gas for Extraction and Refining Springer”. Verlag Ed., p. 246, 1988. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML