-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2016; 6(1): 15-25
doi:10.5923/j.fph.20160601.03

Thin-Layer Chromatography Profiles of Non-Commercial Turmeric (Curcuma longa L.) Products Obtained via Partial Hydrothermal Hydrolysis
Ádina L. Santana, M. Angela A. Meireles
LASEFI/DEA/FEA (School of Food Engineering), UNICAMP (University of Campinas), Brazil
Correspondence to: M. Angela A. Meireles, LASEFI/DEA/FEA (School of Food Engineering), UNICAMP (University of Campinas), Brazil.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study verifies an alternative use of waste turmeric from extraction processes via partial-hydrothermal hydrolysis, which provides partial hydrolyzed-deflavored and depigmented turmeric (PHDDT) and turmeric hydrolysates (TH). Thin-layer chromatography was used to fingerprint and evaluate the chemical compositions of these products in terms of phenolics, antioxidants, volatiles and sugars, which verified the possibility of adding value to these waste products. Reaction temperature variations influenced the quality of the analyzed compounds. Based on a qualitative approach, PHDDT provided a considerable source of bioactive compounds, while TH samples were determined to be a source of low-chain sugars. The production of various sugars and bioactive compounds from non-commercial turmeric represents a promising alternative for future methodologies that can diminish the quantity of agro-waste and improve economic profitability.
Keywords: Turmeric, Partial Hydrolysis, Thin-Layer Chromatography, NP, DPPH, Vanillin, Alpha-Naphthol
Cite this paper: Ádina L. Santana, M. Angela A. Meireles, Thin-Layer Chromatography Profiles of Non-Commercial Turmeric (Curcuma longa L.) Products Obtained via Partial Hydrothermal Hydrolysis, Food and Public Health, Vol. 6 No. 1, 2016, pp. 15-25. doi: 10.5923/j.fph.20160601.03.
Article Outline
1. Introduction
- Turmeric (Curcuma longa L.) is an important medicinal plant and source of phenolic compounds, volatile oil, sugars, proteins and resins [1, 2]. The pigments in the colorant extracts obtained from turmeric are collectively known as curcuminoids, which are phenolic compounds. The major constituent is curcumin (60-80%) in addition to small amounts of demethoxycurcumin (15-30%) and bisdemethoxycurcumin (2-6%) [3].Curcumin is widely used for foods and dyes, and possesses numerous biological benefits, including antioxidant, anti-inflammatory, antimicrobial, antiparasitic, antimutagenic, anticancer and antivirus properties [4].Brazil possesses favorable turmeric cultivation conditions. The dry basis curcuminoid pigment levels in Brazilian turmeric range from 1.4 to 6.1 g/100 g, while the volatile oil fraction varies between 1.0 and 7.6 ml/100 g [5].Several techniques have been used to qualitatively and quantitatively analyze turmeric species and byproducts. High-performance liquid chromatography (HPLC) was used to detect curcuminoid in extracts obtained via pressurized liquid extraction (PLE) [6]. The extracts were then used to standardize a beauty cream formulated with turmeric powder [7]. The curcumin contents of various curcuma varieties collected from different regions of India have been analyzed [2]. Random Amplified Polymorphic DNA was used to detect adulterants in the Curcuma zeodoaria and Curcuma malabarica species [1]. Thin Layer Chromatography (TLC) was used to identify curcumin in curcuma varieties [8], and turmeric extracts were obtained by supercritical fluid extraction (SFE), low-pressure solvent extraction (LPSE) and hydrodistillation [9].TLC is one of the easiest and most versatile methods for identifying and separating compounds due to its low cost, simplicity, short development time, high sensitivity and good reproducibility [10].TLC simultaneously separates substances in space. The RF (retardation factor, ratio of fronts or retention index) value is the standard measure of retention. Given the general difficulty of controlling absolute RF values, it is common to separate standards and samples in the same system for identification purposes [11].SFE has been used to extract volatile oils [12], and turmeric curcuminoids have been obtained using pressurized liquid extraction (PLE) with ethanol [6]. This study uses partial-hydrothermal hydrolysis to obtain new products from deflavored and depigmented turmeric (DDT) using pressurized water; DDT is the residue of the SFE and PLE processes. A partial-hydrothermal hydrolysis was performed to generate two main products: the partial-hydrolyzed deflavored and depigmented turmeric (PHDDT), which is a mixed biopolymer, and turmeric hydrolysate (TH), which is the liquid fraction source of low-chain sugars.Few studies exist regarding the potential use of turmeric rhizomes after the deodorization and depigmenting processes. In most of cases, the material is not reused, increasing the amount of environmental waste [13]. Processes are needed that can generate added-value products from food waste, which can be used for feed, cosmetics, energy and pharmaceuticals. This study qualitatively determines the chemical composition of PHDDT and TH obtained via the partial-hydrothermal hydrolysis of DDT using TLC.Standards were used to assist in the identification of compounds present in the extracts when known compounds were present in the mixture, including phenolics, antioxidants, volatile oil compounds and sugars. The theoretically known compounds presented in the samples were identified via RF calculations of the separated zones. The resulting RF values of the samples were compared to the RF values of the standards.
2. Materials and Methods
2.1. Material
- Deflavored and depigmented turmeric (DDT) was obtained from the crude raw material purchased from the Oficina de Ervas Farmácia de Manipulação Ltda (lot 065DM, Ribeirão Preto, Brazil), from which volatile oil was removed using supercritical CO2 at 60°C and 250bar, while curcuminoids were removed using pressurized ethanol at 60°C and 100bar [6].
2.2. Experimental Methods
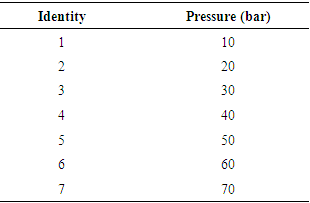
- Partial hydrolysis procedures were performed in a home-made PLE apparatus, shown in Figure 1. Three temperature levels (40, 70 and 100°C) and seven pressure levels (10, 20, 30, 40, 50, 60 and 70bar) were used.
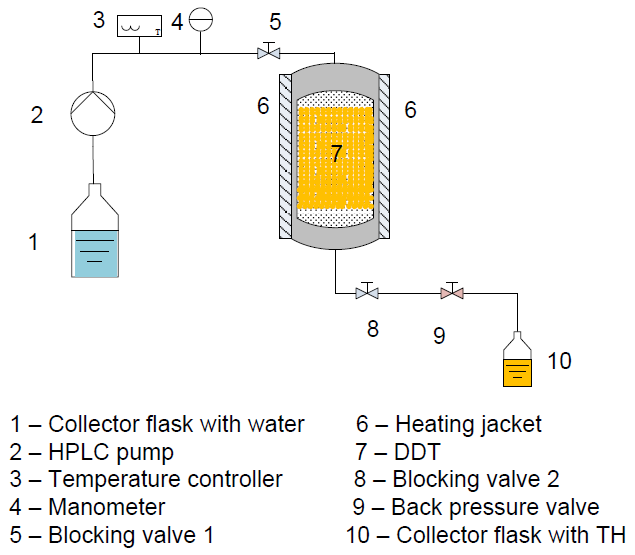 | Figure 1. Home-made PLE system |
2.3. Thin Layer Chromatography
- Silica gel plates with aluminum backs were used as the stationary phase, including ultraviolet light-sensitive (UV254, Alugram®, Xtra SIL G, Macherey-Nagel, Germany) and non-UV-sensitive plates (Alugram®, Xtra SIL G, Macherey-Nagel, Germany) with 10 cm × 10 cm dimensions. The TLC plates were prepared by establishing a 1 cm distance from the origin, 8 cm solvent travel distance and 1 cm distance from the solvent front.Approximately 10 μL of the samples were spotted on the TLC plates using capillary glass tubes with an approximately 1 cm distance from each band. The plates were then developed in glass chambers via mobile phase elution.The bands of compounds generated by the constituents that could not be detected in the visible region were visualized using a UV (Multiband UV – 254-366 nm, UVGL-58, Mineralight® Lamp, Upland, CA, EUA) equipped with a cabinet (UVP-Chromato-VUE, CC-10, Upland, CA, EUA) for short wavelength (254 nm) and long wavelength (366 nm) analyses.
2.3.1. Sample Preparation and Identification
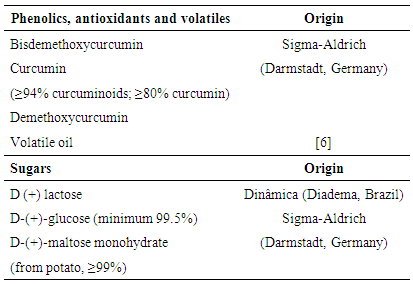
- Ethanol was used as solvent to identify phenolics, antioxidants and volatiles because bioactive turmeric compounds are insoluble in water and soluble in organic solvents, such as ethanol, ethyl acetate and acetone. The standards used to identify these PHDDT and TH compounds included curcumin, demethoxycurcumin, bisdemethoxycurcumin and turmeric volatile oil, which were obtained from the SFE process (Table 1). These standards were diluted in ethanol until a concentration of 15 mg/ml was reached. All solvents and chemicals were of analytical grade.PHDDT samples were diluted in ethanol until the adequate concentration (30 mg/ml) was reached. TH samples were filtrated and concentrated using a freeze-dryer (L101, Liobras, São Carlos, Brazil). The lyophylizated TH samples were diluted in ethanol until a final concentration of 30 mg/ml was reached.PHDDT dilutions were made to identify sugars using Milli-Q water (EMD Millipore Corporation, Merck, Darmstadt, Germany) at a concentration of 200 mg/ml. Liquid TH was used without any further treatment. The glucose, maltose and lactose standards (Table 1) were diluted in water until a concentration of 10 mg/ml was reached.
|
|
2.3.2. Detection
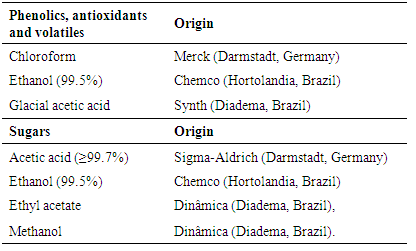
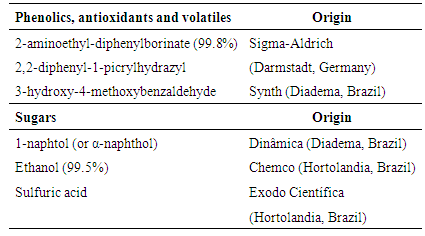
- The phenolics were detected using an NP (2-aminoethylborinate) spray reagent, according to the Wagner and Bladt [14], which was adapted by Albuquerque et al. [16].Antioxidant compounds were detected by spraying a DPPH solution, in which 0.5 g of DPPH (2, 2-diphenyl-1-picrylhydrazyl) was diluted in 250 ml of methanol [16].Volatiles were detected by spraying a sulfuric vanillin (SV) reagent, using the formulation suggested by Krishnaswamy [17], in which 0.5 g of vanillin (3-hydroxy-4-methoxybenzaldehyde) was diluted in 20 ml of ethanol and 80 ml of sulfuric acid. Before and after spraying with NP, DPPH and SV reagents, the plates were inserted into a UV chamber to visualize the compound bands that could not readily be observed.The sugar detection methodology of Lewis and Smith [18], which was adapted by Fried and Sherma [15], was used. A 0.1 M sodium bisulfite (Dinâmica, Diadema, Brazil) solution was used to impregnate the plates and facilitate the resolution of the samples in the mobile phase. This solution was formulated by diluting 10.4 g of sodium bisulfite in 1 liter of Milli-Q water. The TLC plates were then pre-washed in a glass chamber containing this solution, air dried and activated in an oven (Tecnal, TE-385-1, Piracicaba, São Paulo) at 100°C for 30 minutes prior to spotting.Carbohydrates are extremely hydrophilic compounds. Therefore, they strongly attach to adsorbents, such as silica gel, alumina and cellulose. Thus, highly polar solvents were necessary in the mobile phase of TLC development [18].The sugar mobilities on silica gel primarily depend on the molecular weights and the number of hydroxyl groups. The resolution is improved by impregnating silica gel with weak acid salts or via the use of cellulose layers [18].A sulfuric α-naphthol (SAN) reagent was used to detect sugars. This reagent was prepared by adding 5 g of α-naphthol to 33 ml of ethanol (solution A). Then, 21 ml from solution A were combined with 81 ml of ethanol, 8 ml of water and 13 ml of sulfuric acid. After spraying, the plates were heated for approximately 5 minutes at 100°C. The visualizing reagents (spray solutions) were formulated using the reagents listed in Table 3.
|
3. Results and Discussion
3.1. Standards Fingerprints
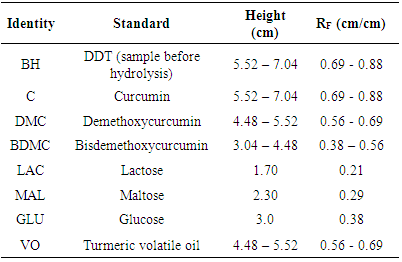
- A sufficiently strong TLC solvent causes a sample to shift into the RF range of 0.2–0.8. In addition, if the correct selectivity is attained, the solvent will evenly distribute the sample components throughout this range. If the sample contains a wide range of sample sizes, then the correct mobile phase will ensure the adequate separation of the major and minor components, rather than an even distribution throughout the RF range [19]. Table 4 lists the standards used in this study and their RF values.
|
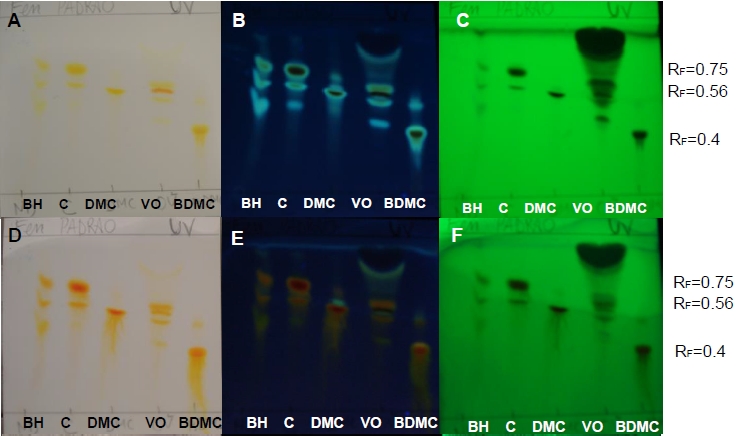 | Figure 2. Curcuminoids standards, turmeric volatile oil and DDT solutions on UV-sensitive plates without a spray reagent (A, B and C) and sprayed with NP (D, E and F) at UV 366 nm and UV 254 nm |
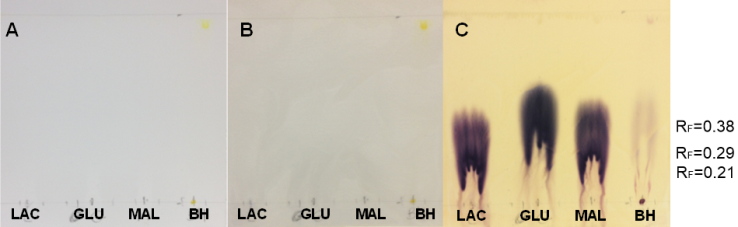 | Figure 5. Sugar standard solutions and DDT samples on non-UV-sensitive plates before being sprayed (A), after being sprayed with SAN (B) and after activation at 100°C (C) |
3.2. Sample Fingerprints and Chemical Composition
- Partial hydrolysis reactions occurred at three temperature levels (40, 70 and 100°C). The sample identities on the plates are listed as a function of the pressure in Table 5.
|
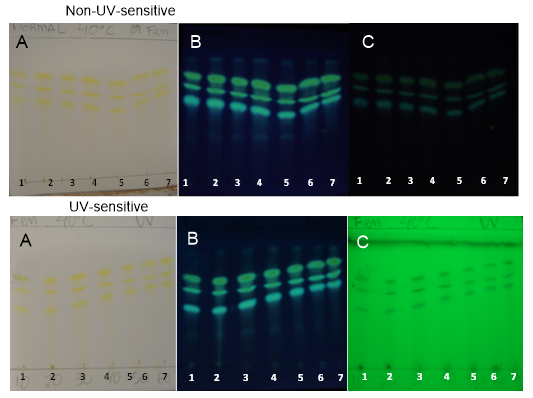 | Figure 6. PHDDT samples obtained at 40°C on non-UV-sensitive and UV-sensitive plates without a spray reagent at visible (A), UV 366 nm (B) and UV 254 nm (C) levels |
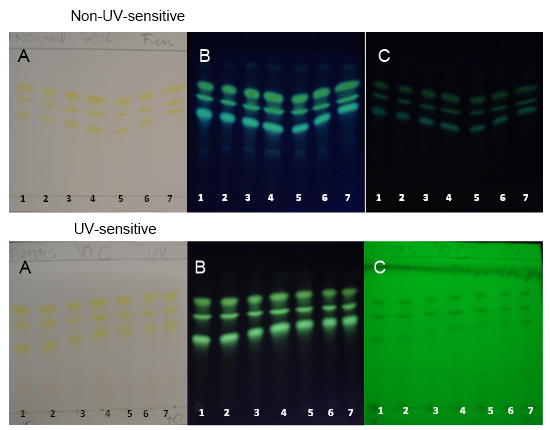 | Figure 7. PHDDT samples obtained at 70°C on non-UV-sensitive and UV-sensitive plates without a spray reagent at visible (A), UV 366 nm (B) and UV 254 nm (C) levels |
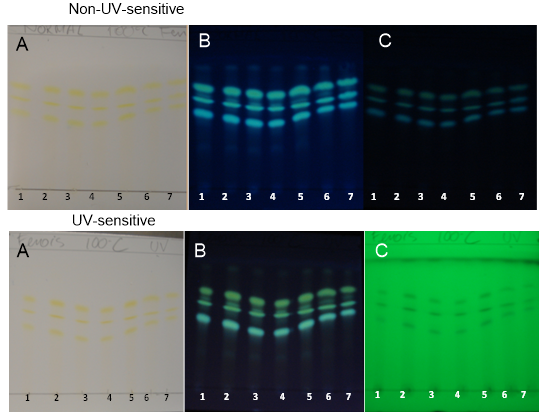 | Figure 8. PHDDT samples obtained at 100°C on non-UV-sensitive and UV-sensitive plates without a spray reagent at visible (A), UV 366 nm (B) and UV 254 nm (C) levels |
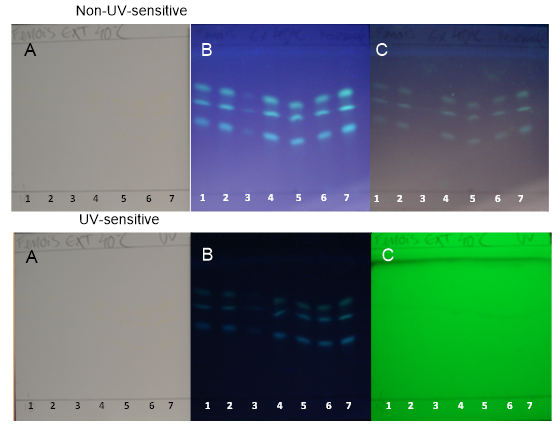 | Figure 9. TH samples obtained at 40°C on non-UV-sensitive and UV-sensitive plates without a spray reagent at visible (A), UV 366 nm (B) and UV 254 nm (C) levels |
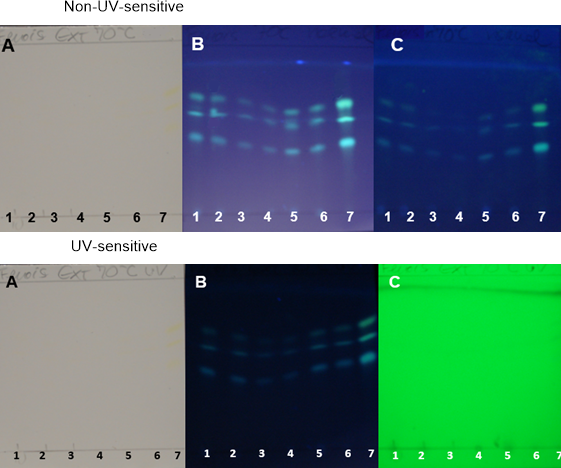 | Figure 10. TH samples obtained at 70°C on non-UV-sensitive and UV-sensitive plates without a spray reagent at visible (A), UV 366 nm (B) and UV 254 nm (C) levels |
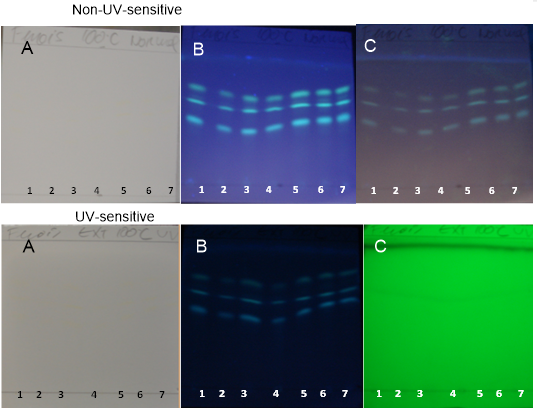 | Figure 11. TH samples obtained at 100°C on non-UV-sensitive and UV-sensitive plates without a spray reagent at visible (A), UV 366 nm (B) and UV 254 nm (C) levels |
3.3. Phenolic Compounds
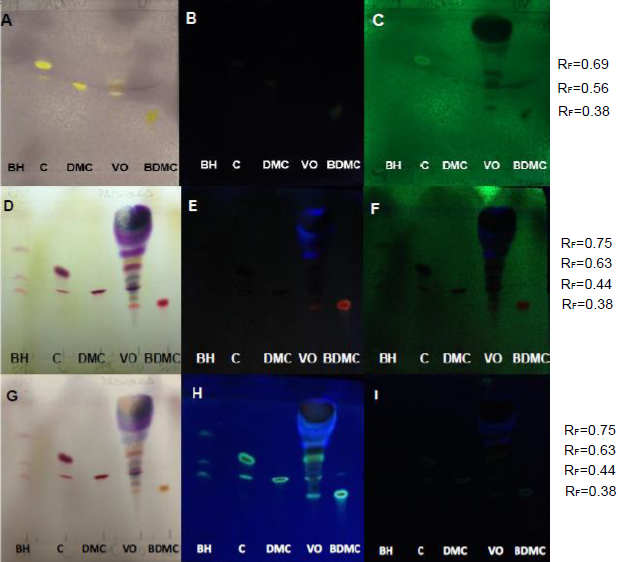
- Curcuminoids and volatile oil are the major bioactive constituents of turmeric. Curcuminoid analyses are essential for determining the quality of the turmeric plant material and processed products [26].PHDDT and TH sample fingerprints displayed dark yellow and light yellow zones in the visible spectrum after being sprayed with NP (Figures 12-17). Dark-yellow to orange spots were visualized on PHDDT samples at 366 nm, while low intensity pale-yellow spots were visualized on TH samples at 366 nm.
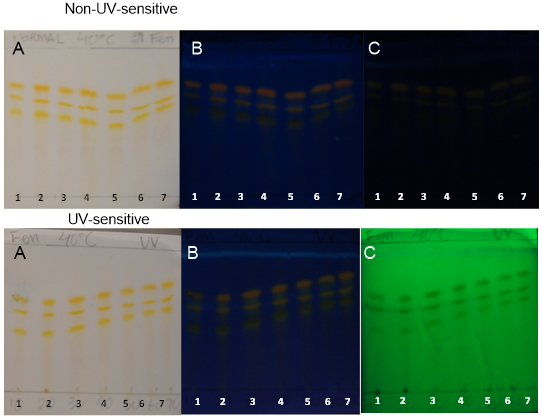 | Figure 12. PHDDT samples after hydrolysis at 40°C on non-UV-sensitive and UV-sensitive plates sprayed with NP at visible (A), UV 366 nm (B) and UV 254 nm (C) levels |
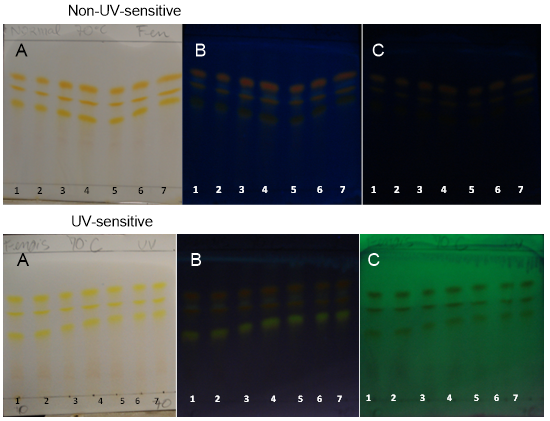 | Figure 13. PHDDT samples obtained at 70°C on non-UV-sensitive and UV-sensitive plates sprayed with NP at visible (A), UV 366 nm (B) and UV 254 nm (C) levels |
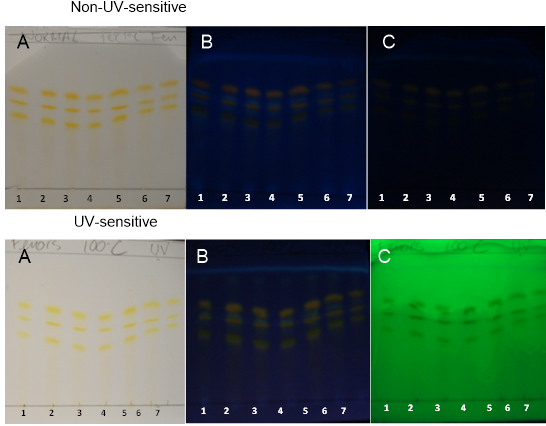 | Figure 14. PHDDT obtained at 100°C on non-UV-sensitive and UV-sensitive plates sprayed with NP at visible (A), UV 366 nm (B) and UV 254 nm (C) levels |
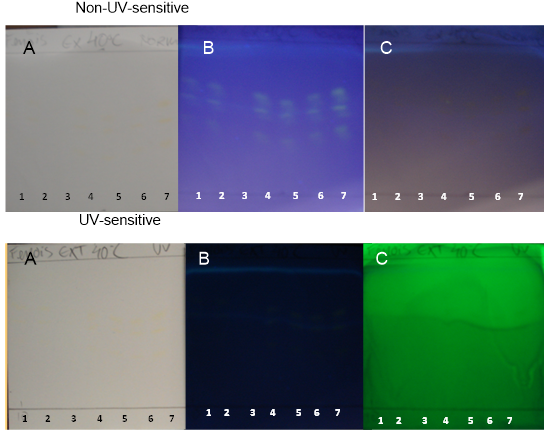 | Figure 15. TH obtained at 40°C on non-UV-sensitive and UV-sensitive plates sprayed with NP at visible (A), UV 366 nm (B) and UV 254 nm (C) levels |
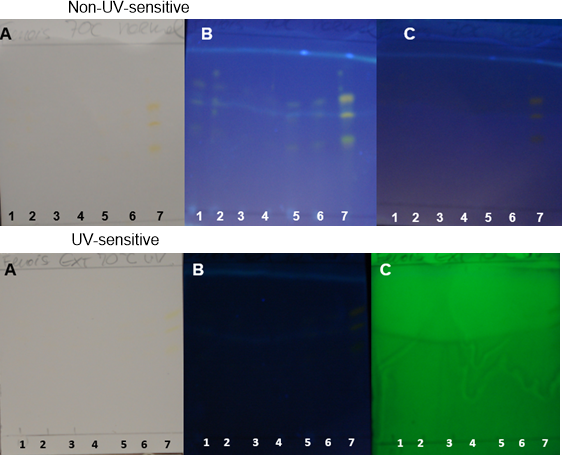 | Figure 16. TH obtained at 70°C on non-UV-sensitive and UV-sensitive plates sprayed with NP at visible (A), UV 366 nm (B) and UV 254 nm (C) levels |
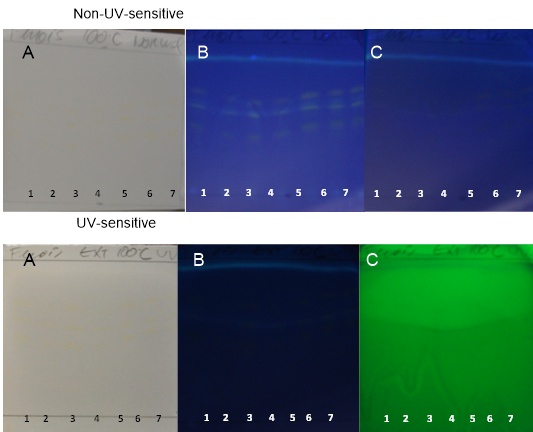 | Figure 17. TH samples obtained at 100°C on non-UV-sensitive and UV-sensitive plates sprayed with NP at visible (A), UV 366 nm (B) and UV 254 nm (C) levels |
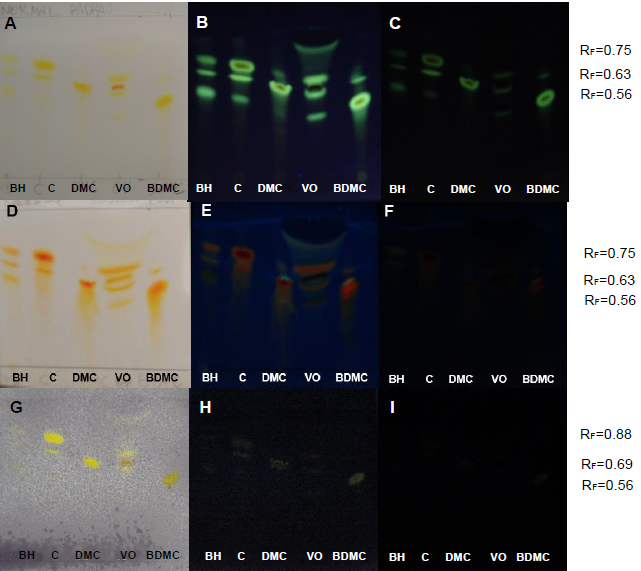
3.4. Antioxidant Compounds
- Antioxidant agents can be used for several cosmetic and medicinal applications [29]. Free radical scavenging compounds appeared as yellowish spots against a purple background, indicating a positive antioxidant activity due to the presence of an active antioxidant [30].The TH samples were not visualized before or after DPPH detection at all temperature or pressure conditions and independent of the type of TLC plate used.The PHDDT samples were not detected using UV-sensitive plates after being sprayed with DPPH. The antioxidant strength of PHDDT (Figures 18, 19 and 20) on UV-sensitive plates is classified as a weak activity. The antioxidant composition varied as the temperature increased.The behaviors observed in the DDT and TH samples vary from the behaviors exhibited by other raw materials, such as bamboo leaves [31] and German Propolis [32], whose constituents were intensively detected by a DPPH solution and exhibited strong antioxidant activities.
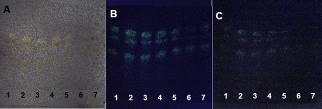 | Figure 18. PHDDT samples obtained at 40°C on non-UV-sensitive plates sprayed with DPPH at visible (A), UV 366 nm (B) and UV 254 nm (C) levels |
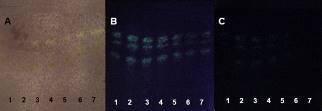 | Figure 19. PHDDT samples obtained at 70°C on non-UV-sensitive plates sprayed with DPPH at visible (A), UV 366 nm (B) and UV 254 nm (C) levels |
 | Figure 20. PHDDT samples obtained at 100°C on non-UV-sensitive plates sprayed with DPPH at visible (A), UV 366 nm (B) and UV 254 nm (C) levels |
3.5. Volatile Compounds
- The SV can be used to detect steroids, volatile oils, terpenes, carotenoids, phenols, catechins, flavonoids, ginsenosides, fatty acids and antibiotics [33]. The SV fingerprints were detected using UV-sensitive plates. PHDDT sample bands were only visualized at the visible level (Figures 21 and 22). TH samples were not visualized at the visible level or by UV-light after elution and being sprayed with SV.Phenolic compounds with aromatic structures display intense absorption in the UV region of the spectrum associated with green, yellow, white to pale yellow, purple, pink, red, blue, grey, brown or black spots [34].Dark red spots immediately appeared on DDT samples after being sprayed with SV (Figure 21) at RFs of 0.56-0.75, while light-red spots appeared at 0.44-0.5. The intensity of the coloration partially disappeared after 30 minutes (Figures 22A, 22B and 22C).
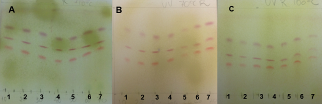 | Figure 21. PHDDT samples obtained at 40 (A), 70 (B) and 100°C (C) after being sprayed with SV at the visible level |
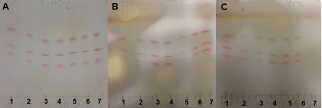 | Figure 22. PHDDT samples obtained at 40 (A), 70 (B) and 100°C (C) 30 minutes after being sprayed with SV at the visible level |
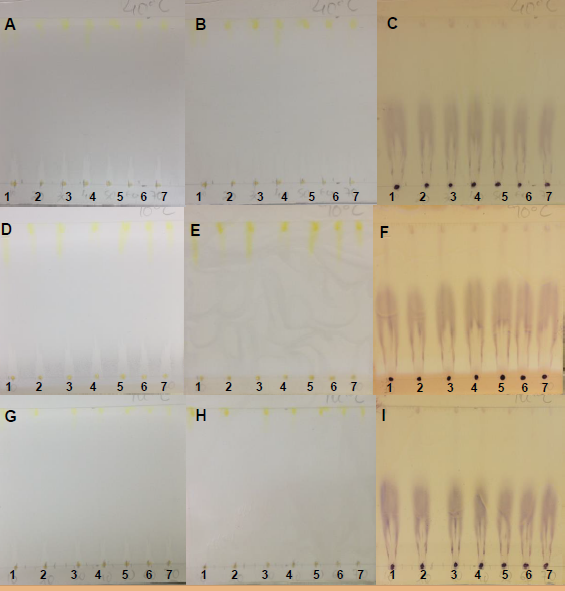
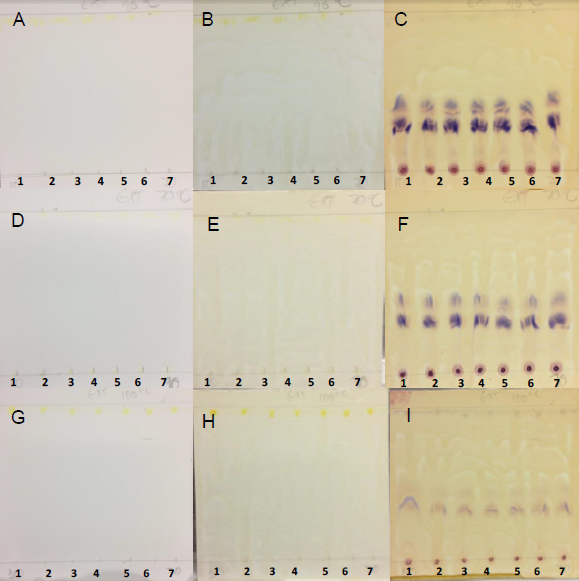
3.6. Sugars
- The sugars detection via TLC uses furfuraldehyde in conjunction with numerous phenolic reagents, which give rise to different colors, e.g., α-naphtol yields a blue-violet coloration. The ketone and aldehyde groups of the reducing sugars preferentially react [35].Carbohydrates attach to adsorbents. Thus, the mobile phase must be extremely polar. Buffering the silica gel with weak salt acids enhances the monosaccharide separation during TLC [35].Band separation did not occur on PHDDT samples (Figure 23), because the material is a source of high-molecular- weight sugars, such as starch [36].
4. Conclusions
- Turmeric is an important plant that is used as a medicine, condiment and cosmetic due to its wide range of bioactive substances. However, few studies have evaluated the functionality and processing feasibility of deflavored and depigmented turmeric.This study explores a method that reuses waste turmeric from extraction processes via partial hydrothermal hydrolysis, generating partial-hydrolyzed deflavored and depigmented turmeric and turmeric hydrolysates products. The chemical profiles of these products were evaluated using thin layer chromatography, which analyzed phenolics, antioxidant, volatiles and sugars.According to the TLC fingerprints, PHDDT and TH samples are predominant in phenolic compounds, presenting light-blue and light-green fluorescences at 366 nm and 254 nm without a spray reagent.Establishing a comparison between these two products, PHDDT samples provide the most bioactive compounds, including curcuminoids, antioxidants and volatiles. However, TH is a considerable source of low-chain sugars when submitted to partial hydrolysis at 40°C.Finally, this studied showed that waste turmeric can be reused for subsequent processes that generate new products with promising applicabilities.
ACKNOWLEDGEMENTS
- The authors acknowledge financial support from CNPq (470916-2012-5). Ádina L. Santana thanks CAPES (2952/2011) for her PhD assistantship. M. Angela A. Meireles thanks CNPq productivity grant (301301/2010-7).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML