-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2015; 5(6): 220-228
doi:10.5923/j.fph.20150506.03

Products, Ice and Water: Risk Factors in a Fishing Company from the Amazon Region
Consuelo L. Sousa 1, José A. Freitas 2, Lúcia F. H. Lourenço 1, Eder A. F. Araujo 1, Maria Regina S. Peixoto Joele 3
1Food Science and Technology Program, College of Food Engineering Federal, University of Para – UFPA, Brazil
2Postgraduate Animal Sciense Program, Federal University of Para – UFPA, Brazil
3Federal Institute of Education, Science, and Technology of Para, IFPA, Castanhal, PA, Brazil
Correspondence to: Consuelo L. Sousa , Food Science and Technology Program, College of Food Engineering Federal, University of Para – UFPA, Brazil.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This paper aimed to evaluate the risk potential for frozen fish, ice and water in a fishing company. For the fish samples it was carried out microbiological and physicochemical analyses. In ice and water was carried out microbiological analyses. Amongst the pathogenic bacteria, only coagulase-positive staphylococci has been detected in 2.7% of the samples within the limits established by the Brazilian legislation. However, higher counts have been found superior to 2.7 log10 MPN g-1 of coliforms at 45°C and at 7 log10 cfu g-1 to mesophilic and psychrotrophic at 1% and 7% of the samples of fillet, respectively. Values of pH and TVB-N higher than the ones established by the national legislation have been detected at 18.1% and at 3.5% of the samples, respectively. In all the analyzed samples of ice and water it was not detected the presence of coliforms at 35°C and at 45°C.
Keywords: Fishes, Microorganisms, Quality, Fillet, Flitch
Cite this paper: Consuelo L. Sousa , José A. Freitas , Lúcia F. H. Lourenço , Eder A. F. Araujo , Maria Regina S. Peixoto Joele , Products, Ice and Water: Risk Factors in a Fishing Company from the Amazon Region, Food and Public Health, Vol. 5 No. 6, 2015, pp. 220-228. doi: 10.5923/j.fph.20150506.03.
Article Outline
1. Introduction
- The Brazilian production of fishes has grown 13,2% from 2010 to 2011 reaching total of 1,431,974,4 tons, from which 33,000 were destined to exportation. Mainly to the USA, Spain and Japan, being the frozen fishes almost half of this total exported. The state of Pará in the Brazilian Amazon Region has been highlighted in such statistics with its total production estimated in 11% of the national production. Besides, the State of Pará was the only one that has performed the implantation of an industrial production equivalent to 14% of the national industrial production [1].The fish is an important part of the diet in several countries, presenting a low content of cholesterol, a higher digestibility when compared to other animals meat, a source of a high biological value protein with a balance of essential amino acids and a significant reserve of poli-insaturated fatty acids from the Omega 3 and Omega 6 series, which several benefits to the human health are attributed to these fatty acids [2]. As it is a type of food with a high nutrition value, with a pH near neutral and a high activity of water, the fish is quite susceptible to develop microbes, what may cause alterations of the physical or chemical nature of its color, consistency, smell and flavor, besides the risks to the health of consumers as it is a type of food that has been associated to diseases related to the food [3, 4].The fish may be the host of a great number of microorganisms that are pathogenic to the man, originating from the environmental contamination, but also from improper manipulation since its capture to the final consumption [5, 6]. Due to its high perishing, it must be conserved under low temperatures and be manipulated in proper sanitary conditions during all its productive chain so that it may be offered a safe product to the consumer [6].The ice and the water used in all the productive chain of the fish must be fresh as they directly affect the quality of the product. When used properly and in the correct quantity, the ice contributes to the conservation of the fish reducing its temperature to 0 to 2°C, delaying enzymatic and bacterial alterations [4].The hygienic and sanitary conditions of the fish, equipment and staff, the proper control of the cold chain during the process, the quality of the ice and water used in all steps of the process are determining factors in the quality of the final product [7]. Therefore, the application of the micro biological and physical/chemical control together with the tools for the quality management, as Good Manufacturing Practices (GMP) and the System of Hazard Analysis and Critical Control Points (HACCP), inside the industries, are fundamental to the lowering of the risks in the production of safe foods.Considering the relevance that the industrial fishing activity of the Brazilian Amazon Region has in the world scenery and the importance of the production and consumption of safe foods, this paper had the aim to evaluate the potential of risk of frozen fish, ice and water, in a fishing industry through microbiological and physicochemical analyses.
2. Materials and Methods
2.1. Sample Collecting
- It was collected, from the period of March, 2010 to November, 2011, 144 samples of frozen fish, 12 samples of ice and 24 samples of water in an industry of fish processing located in the Northeast of the State of Pará. From the total of fish samples, 110 were fillets from different species, being 13 from timbiro (Oligoplites saurus), 54 from pescada gó (Macrondon ancylodon), 17 from piramutaba (Brachyplatystoma vaillanti), 14 from uritinga (Arius proops) and 12 from bandeirado (Bagre marinus). The other 34 samples were from flitch of piramutaba (Brachyplatystoma vaillanti). All samples were collected in triplicate sets in the primary poli-propylene bag used for trading, containing 1 kg of fish. The ice and water were collected in sterile bags of poli-propylene with a capacity of approximately 200 mL and containing 1 mL of a solution of tio-sulfate of sodium at 0.25%. The 12 samples of ice were collected directly from the silo and the samples of water were collected from two distinct places: 12 samples collected directly from the faucet that had been used for the cleaning of hands in the reception room and 12 samples collected directly from the faucet of the production site, used for the fish cleaning. Such faucets were cleaned with alcohol (70%) and flamed before each collection. After the collection, all samples were placed in isothermal bags with ice and transferred to the laboratory.
2.2. Microbiological Analyses of Fishes
- All fish samples, after thawing under refrigeration (4°C) were subjected to analysis: Salmonella spp, coagulase positive staphylococci, Escherichia coli, coliforms at 45° and total aerobic mesophilic and psychrotrophic. The analytical methodology used is described in the Compendium of Methods for the Microbiological Examination of Foods [8] and all analyzes were carried out in triplicate.Briefly, 25 grams of fish were weighed into sterile stomacher bag (Seward Medical, London, UK) and 225 mL of buffered peptone broth were added, and the mixture was homogenized for 60 s in a stomacher (Lab Blender 400; Seward Medical). Serial dilutions were made in Butterfield’s buffer. For Salmonella spp isolation, the samples were pre-enriched in buffered peptone broth, enriched in, tetrathionate broth (TTB) and Rappaport Vassiliadis broth (RVB) and plated on three selective plates viz., Hektoen enteric agar (HEA), bismuth sulphite agar (BSA) and xylose lysine desoxycholate agar (XLD). Five typical colonies from each of the selective agars were subjected to a battery of biochemical tests for confirmation. All dehydrated media used in this study were procured from BD Difco (Maryland, USA) or else indicated. For counting of coagulase-positive staphylococci, 0.5 mL portion of various dilutions were spread on the surface of preset surface dried Baird–Parker agar (Difco, USA) plates supplemented with potassium tellurite and egg yolk emulsion. Typical colonies were confirmed by coagulase test. The most probable number (MPN) technique was used to determine the level of coliforms at 45°C, in a three tubes series. The confirmed coliform colonies were also assessed for detection of E. coli, using eosin-methylene-blue lactose sucrose agar (Merck, Darmstadt, Germany), then subjected to biochemical tests. Total aerobic mesophilic and psychrotrophic were determined by the pour plate method, using plate count agar (PCA; Oxoid Ltd., London, UK) and incubating at 35°C for 48 h and at 7°C for 10 days, respectively.It was also carried out a search for Listeria spp, using the TECRA® UNIQUE® Listeria kit (TECRA International Pty Ltd, Willoughby, NSW, Australia). The analyses procedures have followed the instruction manual of the manufacturer.
2.3. Physicochemical Analyses of Fishes
- The samples of frozen fish underwent analyses of pH and total volatile basic nitrogen (TVB-N), following the methodology recommended by the Ministry of Agriculture, Livestock and Food Supply [9]. The pH value of the samples was determined through a potentiometer (Hanna Instruments, model HI9321), previously calibrated in tampon solutions of pH 4.0 and 7.0 and the index of TVB-N was determined by the distillation method. All analyses were carried out in triplicate.
2.4. Microbiological Analyses of Ice and Water
- In the samples of ice and water, coliforms counts at 35°C and at 45°C were carried out using the MPN technique of ten multiple pipes together [8]. The samples of ice remained under refrigeration (4°C) until they defrost (10 hours) to be submitted to analysis.
2.5. Statistical Analysis
- The microbiological results expressed in the log basis (coliforms, mesophilic and psychrotrophic) and physicochemical (pH and TVB-N) of the fish samples were submitted to the analysis of variance (ANOVA) and test of means of Turkey (P < 0.05), using Statistica® version 7.0 software.
3. Results and Discussion
3.1. Microbiological Analyses of the Fish
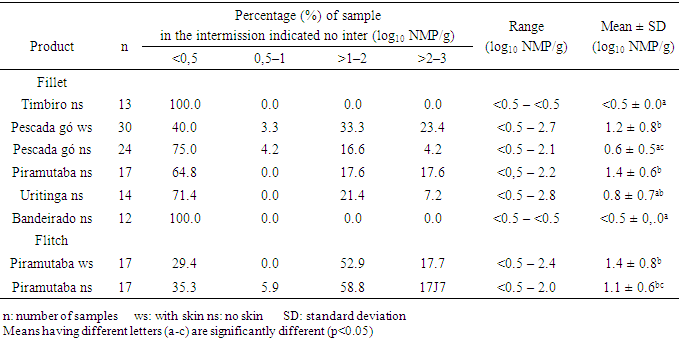
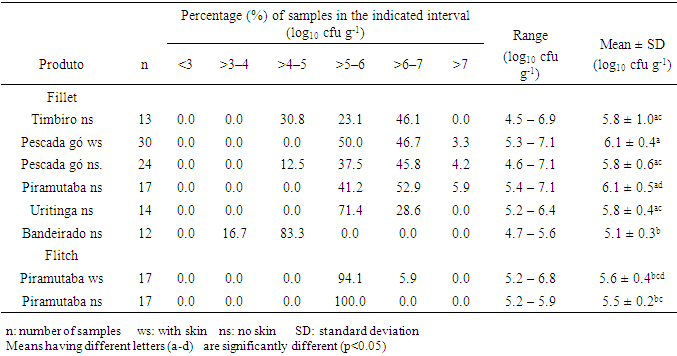
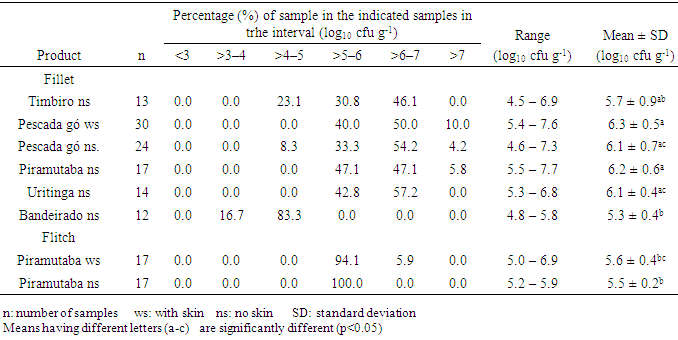
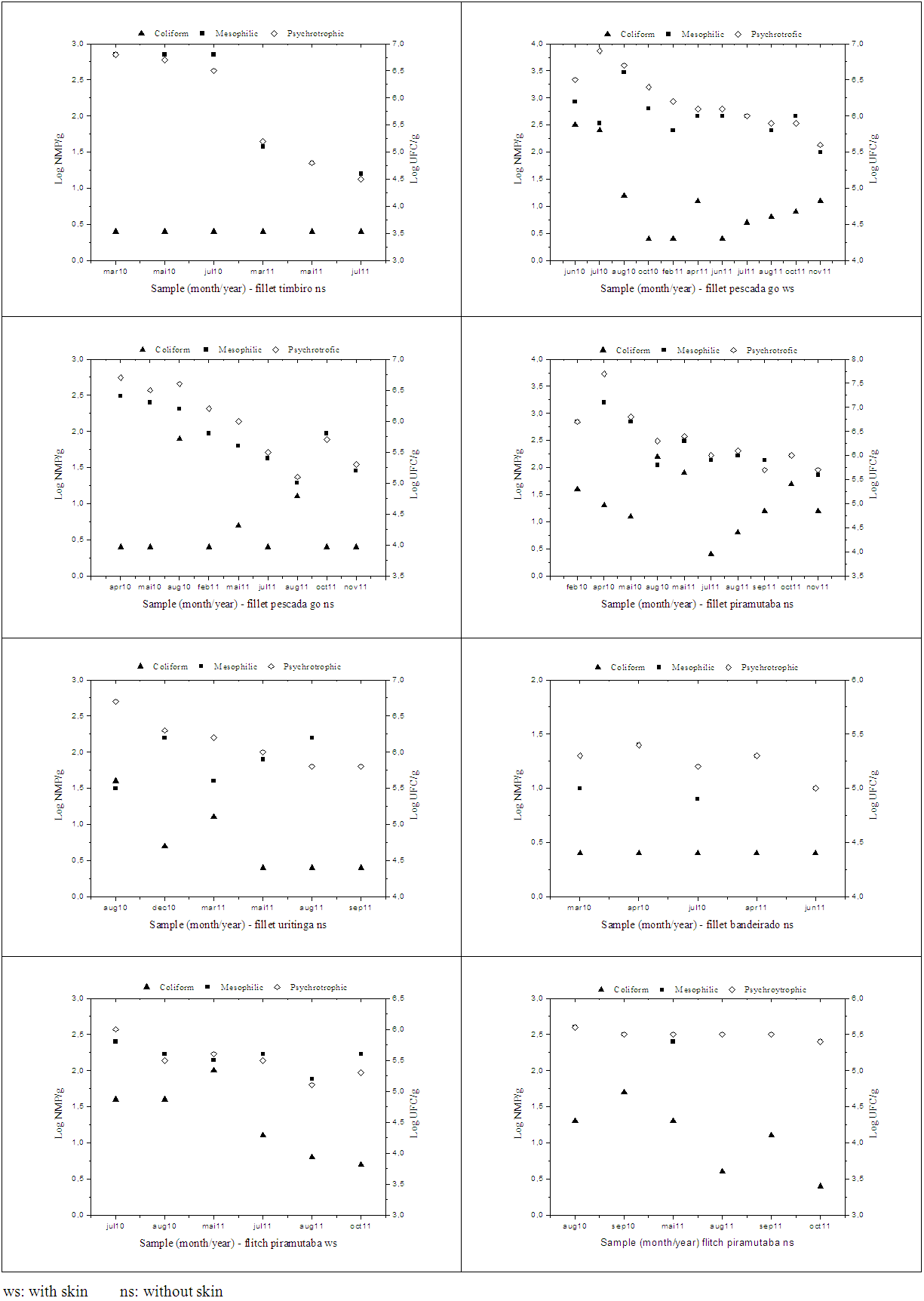
- In all the analyzed samples (fillets and flitch), it was not detected the presence of Salmonella spp, Listeria spp and Escherichia coli. These microorganisms do not take part in the normal microbiology of the fish and, when present in it, they may be associated to the contamination at the capture site, improper manipulation in the productive chain, which include ice, equipment, tools, etc., that may be in contact with the fresh fish [3, 10]. For fish originated from clean water, manipulated hygienically, cross-contamination is the most common cause for the presence of such pathogens [6].Outbreaks of Salmonella associated to fish are rare [10, 11] and in several researches, mainly in processed frozen fish, the absence of Salmonella was reported [7, 12, 13]. However, contamination by Salmonella spp above 10% were mainly detected in fresh and frozen fish traded in free and open markets, as they are more susceptible to cross-contamination due to its constant exposition to environmental factors like: dust, rodents and insects and, therefore, they are considered products with a higher risk to human health [3, 14].L. monocytogenes is highly distributed amongst the animals, men and environment, but, as it is a psychrotrophic pathogen, it is not commonly isolated in tropical waters [15]. In general, L. monocytogenes is not usually found in fishes captured in open waters, however, their contamination may occur in the fishing boats or during their process chain, when contaminated water or ice, surface and tools badly sterilized are used as well as contamination through human or aviary origin [16]. In fishes from coastal areas and from pool cultures that use cattle feces as fertilizers, it is more common the detection of such pathogen [17].E.coli is the classic example of enteric bacterium that causes gastro-enteritis and is frequently isolated from fresh fish (whole or just the fillet), which has its origin in the fecal contamination at the capture place, mainly due to the improper hygienic and sanitary conditions during its process chain and due to the lack of hygiene of the manipulators [11]. However, in frozen fish the absence of E.coli is reported [7, 12].Coagulase-positive staphylococci was found in 2.7% of the samples of fillet analyzed, being two samples of pescada gó with skin and one of piramutaba with variations from 1.3 to 2.6 log10 cfu g-1, considered within the limits of tolerance established by the Brazilian legislation, which is: max. 3 log10 cfu g-1 [18]. Low counts of staphylococcus are acceptable once these microorganisms are not considered good competitors when other bacteria are concerned and, therefore, rarely cause intoxication when present in raw foods, where the normal flora has not been destroyed [11]. The presence of staphylococcus in fish may be mainly associated to a failure during its process chain or improper manipulation of the fish as the bacteria may be present in the nostrils, throats, hair and skin of the human being [5]. Besides, it is well known that many strains do develop slowly in low temperatures, and many of them are resistant to freezing temperatures and most of them being able to survive 20 days at -20°C [10], confirming the importance of its research on frozen fish.In Tables 1 through 3, it is found the results of coliforms at 45°C, aerobic mesophilic and aerobic psychrotrophic in the samples of fish analyzed, respectively. The Brazilian legislation does not provide limits for the counts of such microorganisms in fish, however, but it is well-known that high population levels may reduce the validity span of the product. However, the International Commission on Microbiological Specification for Foods [19] recommends the maximum limit to 2.7 log MPN g-1 for the count of coliforms at 45°C and 7 log10 cfu g-1 to the standard count of aerobic mesophilic and psychrotrophic plaques in fish.
|
|
|
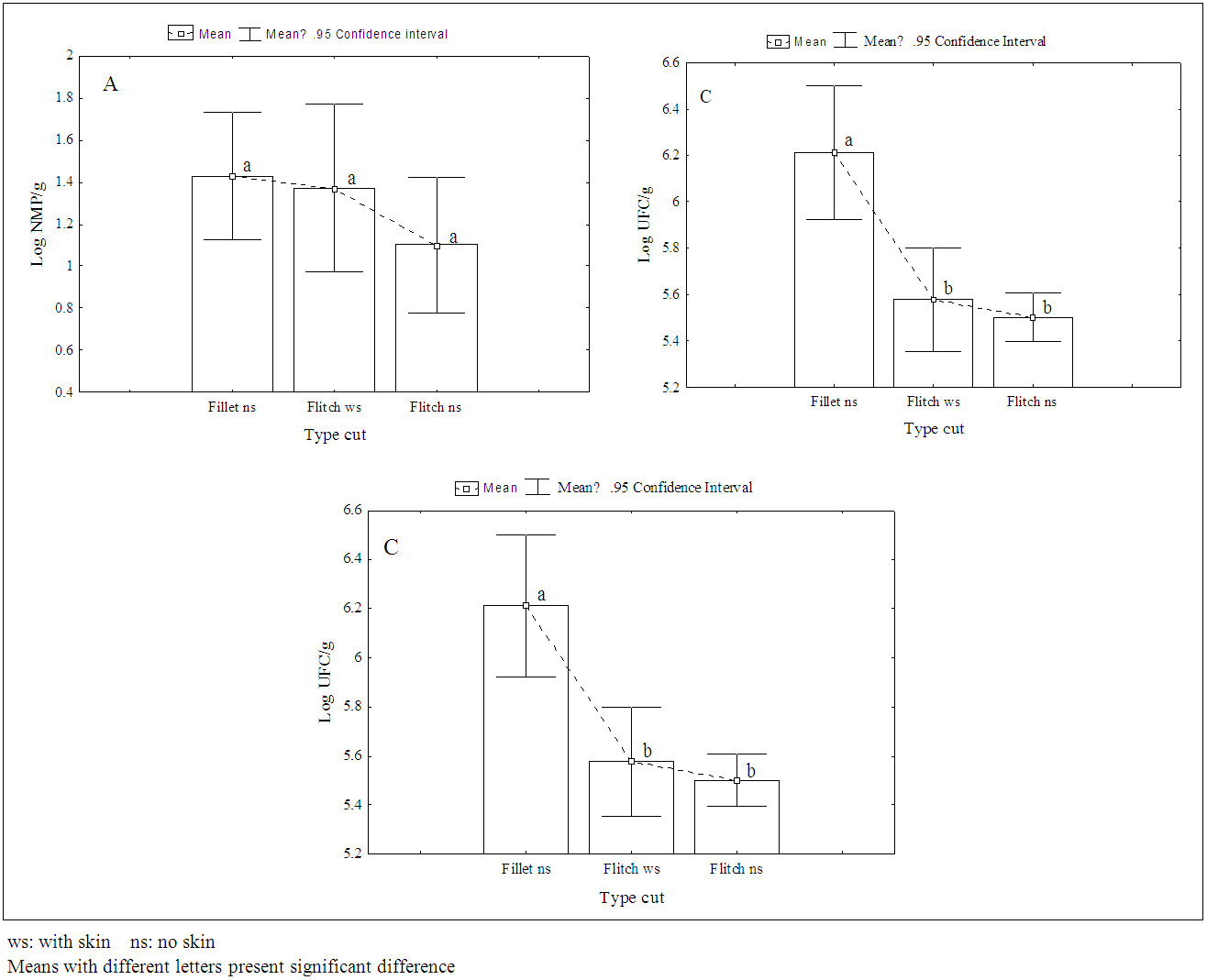 | Figure 1. Comparison of the means of coliforms at 45ºC (A), of mesophilic (B) and psychrotrophic (C) by type of cut in the piramutaba, collected in an industry of Pará (Brazil) |
3.2. Physicochemical Analyses of Fish
- The average values of pH and TVB-N obtained in the samples of frozen fish (fillets and flitch) are in Table 4. The variation of pH and TVB-N was 5.8 to 7.5 and 8.9 to 44.60 mg per 100 g of muscle, respectively. The Regulation of Industrial and Sanitary Inspection of Animal Products [26] establishes that fresh fish, frost or frozen must have in its inner muscles a pH lower to 6.5 and TVB to a maximum of 30.00 mg per 100 g.
|
3.3. Microbial Analyses of Ice and Water
- In all samples of ice and water analyzed it was not detected the presence of coliforms at 35ºC and at 45ºC, being inside the microbial standard of water freshness for human consumption [29] and with proper hygiene / sanitary conditions, being considered without risk to the process and product. Rosas and Reyes [30], when evaluating the microbiological quality of the water used in the industry of fish processing in Venezuela, observed that the water was proper as to the standards of freshness, concluding that the water used did not pose a risk to the process.That the use of chlorine added ice is effective in the reduction of aerobic mesophilic and psychrotrophic microorganism counts in the muscle of the fish [31]. However, the ice used in the conservation of fish is not always presenting a satisfactory quality, due to the presence of microorganisms, generally in great quantities, being able to lead to the contamination of the product during freezing [32].
4. Conclusions
- The samples of fillet and flitch of fish analyzed do not offer any risk to the consumers’ health as for the pathogens, as it was only detected positive coagulasis staphylococcus, however, within the limits recommended by the national legislation. The ice and the water presented a bacteriological pattern of freshness. However, high counts of coliforms at 45ºC, aerobics mesophilic and psychrotrophic and values of pH and contents of TVB-N over the specifications, detected in some samples indicated unsatisfactory quality and conditions for the multiplication of pathogens. The contamination observed may be associated to the improper conditions of hygiene in the steps of processing that may reflect in the commercial life of the product and, therefore, signalize in the direction of Good Practices of Manufacturing and implantation of a system of Analyses of Dangers and Critical Points of Control in the Industry.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML