| [1] | Bortnowska, G., 2010, "Influence of Thermodynamic and Kinetic Factors on the Retention and Release of Aroma Compounds in Liquid Food Systems – A Review", Polish Journal of Food and Nutrition Sciences, 60, (4), 301-307. |
| [2] | Sevenich, R., Bark, F., Kleinstueck, E., Crews, C., Pye, C., Hradecky, J., Reineke, K., Lavilla, M., Martinez-de-Maranon, I., Briand, J. C., Knorr, D., 2015, "The impact of high pressure thermal sterilization on the microbiological stability and formation of food processing contaminants in selected fish systems and baby food puree at pilot scale", Food Control, 50, (539-547), |
| [3] | Guignon, B., Rey-Santos, I., Sanz, P. D., 2014, "Determination, analysis and prediction of the volumetric behavior of milk at high pressure", Food Research International, 64, 336-347. |
| [4] | Medina-Meza, I. G., Barnaba, C., Barbosa-Cánovas, G. V., 2014, "Effects of high pressure processing on lipid oxidation: A review", Innovative Food Science & Emerging Technologies, 22, 1-10. |
| [5] | Athès, V., Paricaud, P., Ellaite, M., Souchon, I., Fürst, W., 2008, "Vapour–liquid equilibria of aroma compounds in hydroalcoholic solutions: Measurements with a recirculation method and modelling with the NRTL and COSMO-SAC approaches", Fluid Phase Equilibria, 265, (1–2), 139-154. |
| [6] | Gironi, F., Maschietti, M., 2012, "Phase equilibrium of the system supercritical carbon dioxide–lemon essential oil: New experimental data and thermodynamic modelling", The Journal of Supercritical Fluids, 70, 8-16. |
| [7] | Setianto, W. B., Yoshikawa, S., Smith Jr, R. L., Inomata, H., Florusse, L. J., Peters, C. J., 2009, "Pressure profile separation of phenolic liquid compounds from cashew (Anacardium occidentale) shell with supercritical carbon dioxide and aspects of its phase equilibria", The Journal of Supercritical Fluids, 48, (3), 203-210. |
| [8] | Solaesa, Á. G., Bucio, S. L., Sanz, M. T., Beltrán, S., Rebolleda, S., 2013, "Liquid–liquid equilibria for systems glycerol + sardine oil + tert-alcohols", Fluid Phase Equilibria, 356, 284-290. |
| [9] | Ferrentino, G., Balaban, M. O., Ferrari, G., Poletto, M., 2010, "Food treatment with high pressure carbon dioxide: Saccharomyces cerevisiae inactivation kinetics expressed as a function of CO2 solubility", The Journal of Supercritical Fluids, 52, (1), 151-160. |
| [10] | Costa, M. C., Krähenbühl, M. A., Meirelles, A. J. A., Daridon, J. L., Pauly, J., Coutinho, J. A. P., 2007, "High pressure solid–liquid equilibria of fatty acids", Fluid Phase Equilibria, 253, (2), 118-123. |
| [11] | Brunner, G., 2015, "Supercritical process technology related to energy and future directions – An introduction", The Journal of Supercritical Fluids, 96, 11-20. |
| [12] | Fonseca, J. M. S., von Solms, N., 2012, "Development and testing of a new apparatus for the measurement of high-pressure low-temperature phase equilibria", Fluid Phase Equilibria, 329, 55-62. |
| [13] | Galia, A., Argentino, A., Scialdone, O., Filardo, G., 2002, "A new simple static method for the determination of solubilities of condensed compounds in supercritical fluids", The Journal of Supercritical Fluids, 24, (1), 7-17. |
| [14] | Peper, S., Dohrn, R., 2012, "Sampling from fluid mixtures under high pressure: Review, case study and evaluation", The Journal of Supercritical Fluids, 66, 2-15. |
| [15] | Narasigadu, C., Naidoo, P., Coquelet, C., Richon, D., Ramjugernath, D., 2013, "A novel static analytical apparatus for phase equilibrium measurements", Fluid Phase Equilibria, 338, 188-196. |
| [16] | Fonseca, J. M. S., von Solms, N., 2014, "Synthetic methods in phase equilibria: A new apparatus and error analysis of the method", The Journal of Supercritical Fluids, 86, 49-56. |
| [17] | Hicks, C. P., A Bibliography of Thermodynamic Quantities for Binary Fluid Mixtures, Chemical Thermodynamics, Vol. 2, M.L. McClahanChemical Society, London, 1978. |
| [18] | Knapp, H., Doering, R., Oellrich, L., Ploecker, U., Prausnitz, J. M., 1981, "Vapor-Liquid Equilibria for Mixtures of Low-Boiling Substances", DECHEMA Chemical Data Serie,6. |
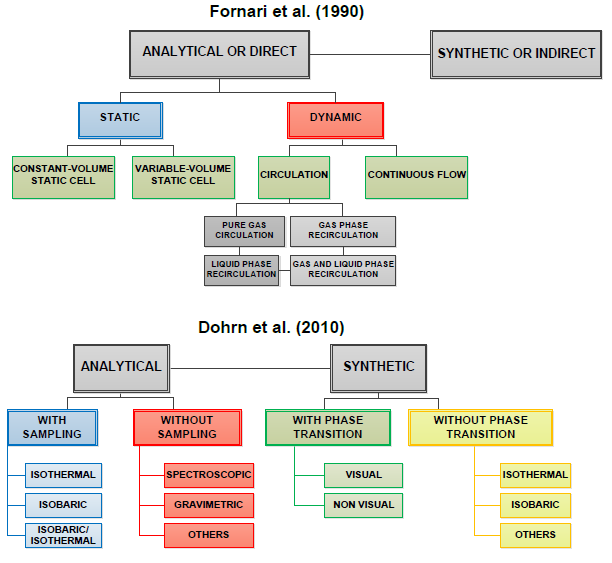
| [19] | Fornari, R. E., Alessi, P., Kikic, I., 1990, "High pressure fluid phase equilibria: experimental methods and systems investigated (1978–1987)", Fluid Phase Equilibria, 57, (1–2), 1-33. |
| [20] | Dohrn, R., Brunner, G., 1995, "High-pressure fluid-phase equilibria: Experimental methods and systems investigated (1988–1993)", Fluid Phase Equilibria, 106, (1–2), 213-282. |
| [21] | Christov, M., Dohrn, R., 2002, "High-pressure fluid phase equilibria: Experimental methods and systems investigated (1994–1999)", Fluid Phase Equilibria, 202, (1), 153-218. |
| [22] | Dohrn, R., Peper, S., Fonseca, J. M. S., 2010, "High-pressure fluid-phase equilibria: Experimental methods and systems investigated (2000–2004)", Fluid Phase Equilibria, 288, (1–2), 1-54. |
| [23] | Fonseca, J. M. S., Dohrn, R., Peper, S., 2011, "High-pressure fluid-phase equilibria: Experimental methods and systems investigated (2005–2008)", Fluid Phase Equilibria, 300, (1–2), 1-69. |
| [24] | Škerget, M., Knez, Ž., Knez-Hrnčič, M., 2011, "Solubility of Solids in Sub- and Supercritical Fluids: a Review", Journal of Chemical & Engineering Data, 56, (4), 694-719. |
| [25] | Robinson, D. B., 1993, "Experimental methods for measurement of phase equilibria at high pressures", Pure & Applied Chemistry, 65, (5), 971-976. |
| [26] | Deiters, U. K., Schneider, G. M., 1986, "High pressure phase equilibria: experimental methods", Fluid Phase Equilibria, 29, 145-160. |
| [27] | Eubank, P. T., Hall, K. R., Holste, J. C., "Review of Experimental Techniques for Vapor-Liquid Equilibria at High Pressures", in Procedings of 2nd International Conference on Phase Equilibria and Fluid Properties in the Chemical Industry, 1981. |
| [28] | Schneider, G. M., Phase equilibria of liquid and gaseous mixtures at high pressures, Le Neindre, B., Vodar, B., Experimental Thermodynamics, Vol. 2, Butterworths, London, 1975. |
| [29] | Tsikilis, D. S., Handbook of Techniques in High-Pressure Research and Engineering, New York, 1968. |
| [30] | Bruin, S., 1999, "Phase equilibria for food product and process design1", Fluid Phase Equilibria, 158–160, (0), 657-671. |
| [31] | Comim, S. R. R., Madella, K., Oliveira, J. V., Ferreira, S. R. S., 2010, "Supercritical fluid extraction from dried banana peel (Musa spp., genomic group AAB): Extraction yield, mathematical modeling, economical analysis and phase equilibria", The Journal of Supercritical Fluids, 54, (1), 30-37. |
| [32] | Saravacos, G. D., Krokida, M. K., Mass transfer properties of foods, CRC Press, Boca Raton, 2014. |
| [33] | Carareto, N. D. D., Costa, M. C., Meirelles, A. J. A., Pauly, J., 2014, "High pressure solid–liquid equilibrium of fatty acid ethyl esters binary systems", Fluid Phase Equilibria, 382, 158-163. |
| [34] | Gros, J. B., Dussap, C. G., 2003, "Estimation of equilibrium properties in formulation or processing of liquid foods", Food Chemistry, 82, (1), 41-49. |
| [35] | Kwok, K., Mauer, J., Taylor, S., 2010, "Phase behavior and moisture sorption of deliquescent powders", Chemical Engineering Science, 21, 5639-5650. |
| [36] | Alavi, T., Pazuki, G., Raisi, A., 2014, "Solubility of fructose in water-ethanol and water-methanol mixtures by using H-bonding models", Journal of Food Science, 79, (5), 839-848. |
| [37] | Philippe, E., Seuvre, A. M., Colas, B., Langendroff, V., Schippa, C., Voilley, A., 2003, "Behavior of Flavor Compounds in Model Food Systems: a Thermodynamic Stud", Journal of Agricultural and Food Chemistry, 51, (5), 1393-1398. |
| [38] | Mession, J. L., Assifaoui, A., Lafarge, C., Saurel, R., Cayot, P., 2012, "Protein aggregation induced by phase separation in a pea proteins–sodium alginate–water ternary system", Food Hydrocolloids, 28, (2), 333-343. |
| [39] | Abderafi, S., Bounahmidi, T., 1999, "Measurement and estimation of vapor–liquid equilibrium for industrial sugar juice using the Peng–Robinson equation of state", Fluid Phase Equilibria, 162, (1–2), 225-240. |
| [40] | Lerici, C. R., Manzocco, L., Anese, M., 1999, "Ethanol in food: liquid–vapour partition in model systems containing Maillard reaction products", Food Research International, 32, (6), 429-432. |
| [41] | Fornari, T., Hernández, E. J., Reglero, G., 2009, "Solubility of supercritical gases in organic liquids", The Journal of Supercritical Fluids, 51, (2), 115-122. |
| [42] | Ruiz-Rodriguez, A., Fornari, T., Hernández, E. J., Señorans, F. J., Reglero, G., 2010, "Thermodynamic modeling of dealcoholization of beverages using supercritical CO2: Application to wine samples", The Journal of Supercritical Fluids, 52, (2), 183-188. |
| [43] | Robinson, A. L., Ebeler, S. E., Heymann, H., Boss, P. K., Solomon, P. S., Trengove, R. D., 2009, "Interactions between Wine Volatile Compounds and Grape and Wine Matrix Components Influence Aroma Compound Headspace Partitionin", Journal of Agricultural and Food Chemistry, 57, (21), 10313-10322. |
| [44] | Fornari, T., Luna, P., Stateva, R. P., 2010, "The vdW EoS hundred years later, yet younger than before. Application to the phase equilibria modeling of food-type systems for a green technology", The Journal of Supercritical Fluids, 55, (2), 579-593. |
| [45] | Maschietti, M., Pedacchia, A., 2014, "Supercritical carbon dioxide separation of fish oil ethyl esters by means of a continuous countercurrent process with an internal reflux", The Journal of Supercritical Fluids, 86, (0), 76-84. |
| [46] | Asenjo, J. A., Andrews, B. A., 2012, "Aqueous two-phase systems for protein separation: Phase separation and applications", Journal of Chromatography A, 1238, 1-10. |
| [47] | Guichard, E., 2006, "Flavour retention and release from protein solutions", Biotechnology Advances, 24, (2), 226-229. |
| [48] | Urmann, M., Hafner, M., Frech, C., 2011, "Influence of protein and stationary phase properties on protein–matrix-interaction in cation exchange chromatography", Journal of Chromatography A, 1218, (31), 5136-5145. |
| [49] | Oey, I., Lille, M., Van Loey, A., Hendrickx, M., 2008, "Effect of high-pressure processing on colour, texture and flavour of fruit- and vegetable-based food products: a review", Trends in Food Science & Technology, 19, (6), 320-328. |
| [50] | Kobori, C. N., Wagner, R., Padula, M., Rodriguez-Amaya, D. B., 2014, "Formation of volatile compounds from lycopene by autoxidation in a model system simulating dehydrated foods", Food Research International, 63, Part A, 49-54. |
| [51] | Kaushik, N., Kaur, B. P., Rao, P. S., Mishra, H. N., 2014, "Effect of high pressure processing on color, biochemical and microbiological characteristics of mango pulp (Mangifera indica cv. Amrapali)", Innovative Food Science & Emerging Technologies, 22, (0), 40-50. |
| [52] | Viñas, P., Bravo-Bravo, M., López-García, I., Hernández-Córdoba, M., 2013, "Dispersive liquid–liquid microextraction for the determination of vitamins D and K in foods by liquid chromatography with diode-array and atmospheric pressure chemical ionization-mass spectrometry detection", Talanta, 115, (0), 806-813. |
| [53] | Al-Darmaki, N., Lu, T., Al-Duri, B., Harris, J. B., Favre, T. L. F., Bhaggan, K., Santos, R. C. D., 2011, "Solubility measurements and analysis of binary, ternary and quaternary systems of palm olein, squalene and oleic acid in supercritical carbon dioxide", Separation and Purification Technology, 83, (0), 189-195. |
| [54] | Spínola, V., Llorent-Martínez, E. J., Castilho, P. C., 2014, "Determination of vitamin C in foods: Current state of method validation", Journal of Chromatography A, 1369, 2-17. |
| [55] | Vicente, G., Paiva, A., Fornari, T., Najdanovic-Visak, V., 2011, "Liquid–liquid equilibria for separation of tocopherol from olive oil using ethyl lactate", Chemical Engineering Journal, 172, (2–3), 879-884. |
| [56] | Souza, A. T., Corazza, M. L., Cardozo-Filho, L., Guirardello, R., Meireles, M. A. A., 2004, "Phase Equilibrium Measurements for the System Clove (Eugenia caryophyllus) Oil + CO2", Journal of Chemical & Engineering Data, 49, (2), 352-356. |
| [57] | Raeissi, S., Peters, C. J., 2005, "Experimental determination of high-pressure phase equilibria of the ternary system carbon dioxide + limonene + linalool", The Journal of Supercritical Fluids, 35, (1), 10-17. |
| [58] | Oliveira, A. L., Melo, V. L. S., Guimarães, A. R., Cabral, F. A., 2010, "Modelling of high-pressure phase equilibrium in systems of interest in the food engineering field using the Peng-Robinson Equation of State with two different mixing rules", Journal of Food Process Engineering, 33, (0), 101-116. |
| [59] | Gironi, F., Maschietti, M., 2008, "Continuous countercurrent deterpenation of lemon essential oil by means of supercritical carbon dioxide: Experimental data and process modelling", Chemical Engineering Science, 63, (3), 651-661. |
| [60] | Druaux, C., Voilley, A., 1997, "Effect of food composition and microstructure on volatile flavour release", Trends in Food Science & Technology, 8, (11), 364-368. |
| [61] | Sahin, S., Sumnu, S., Thermal Properties of Foods, Physical Properties of Foods, Springer New York, 2006. |
| [62] | Otero, L., Ousegui, A., Guignon, B., Le Bail, A., Sanz, P. D., 2006, "Evaluation of the thermophysical properties of tylose gel under pressure in the phase change domain", Food Hydrocolloids, 20, (4), 449-460. |
| [63] | Sharma, A., Tyagi, V. V., Chen, C. R., Buddhi, D., 2009, "Review on thermal energy storage with phase change materials and applications", Renewable and Sustainable Energy Reviews, 13, (2), 318-345. |
| [64] | Joback, K. G., Reid, R. C., 1987, "Estimation of pure-component properties from group-contributions", Chemical Engineering Communications, 57, (1-6), 233-243. |
| [65] | Constantinou, L., Gani, R., 1994, "New group contribution method for estimating properties of pure compounds", AIChE Journal, 40, (10), 1697-1710. |
| [66] | Rao, M. A., Rizvi, S. S. H., Engineering Properties of Food, 2nd ed., Marcel Dekker, Inc., New York, 1995. |
| [67] | Baik, O. D., Marcotte, M., Sablani, S. S., Castaigne, F., 2001, "Thermal and Physical Properties of Bakery Products", Critical Reviews in Food Science and Nutrition, 41, (5), 321-352. |
| [68] | Choi, Y., Okos, M. R., Effects of temperature and composition on the thermal properties of foods, Elsevier, London, 1986. |
| [69] | Alsmeyer, F., Marquardt, W., Olf, G., 2002, "A new method for phase equilibrium measurements in reacting mixtures", Fluid Phase Equilibria, 203, (1–2), 31-51. |
| [70] | Adami, R., Schuster, J., Liparoti, S., Reverchon, E., Leipertz, A., Braeuer, A., 2013, "A Raman spectroscopic method for the determination of high pressure vapour liquid equilibria", Fluid Phase Equilibria, 360, 265-273. |
| [71] | Bogatu, C., Vîlcu, R., Dutţă, A., 2005, "Experimental methods for study high-pressure phase behaviour. Part I. Static methods.", Analele Universităţii din Bucureşti – Chimie Anul XIV (serie nouă), 1-2, (0), 193-203. |
| [72] | Bogatu, C., Vîlcu, R., Dutţă, A., 2006, "Experimental methods for study high-pressure phase behaviour. Part II. Recirculation methods", Analele Universităţii din Bucureşti – Chimie, Anul XV (serie nouă), 1, (0), 57-65. |
| [73] | Bogatu, C., Vîlcu, R., Dutţă, A., 2006, "Experimental methods for study high-pressure phase behaviour. Part III. Continuous flow methods", Analele Universităţii din Bucureşti – Chimie, Anul XV (serie nouă), 2, (0), 27-31. |
| [74] | Subramoney, S. C., Nelson, W. M., Naidoo, P., Coquelet, C., Richon, D., Ramjugernath, D., 2015, "Isothermal vapor–liquid equilibrium data for the ethene + 2,2,3-trifluoro-3-(trifluoromethyl)oxirane binary system between 258 and 308 K at pressures up to 4.5 MPa", Fluid Phase Equilibria, 394, (0), 88-92. |
| [75] | Araus, K. A., del Valle, J. M., Robert, P. S., de la Fuente, J. C., 2012, "Effect of triolein addition on the solubility of capsanthin in supercritical carbon dioxide", The Journal of Chemical Thermodynamics, 51, (0), 190-194. |
| [76] | Mehl, A., Nascimento, F. P., Pessoa, F. L. P., Cardozo-Filho, L., 2011, "Vapor-liquid equilibrium of carbon dioxide + ethanol: experimental measurements with acoustic method and thermodynamic modeling", Journal of Thermodynamics, 1-11. |
| [77] | Subramoney, S. C., Courtial, X., Naidoo, P., Coquelet, C., Richon, D., Ramjugernath, D., 2013, "Isothermal vapor–liquid equilibrium data for the ethylene + 1,1,2,3,3,3-hexafluoro-1-propene binary system between 258 and 308 K at pressures up to 4.56 MPa", Fluid Phase Equilibria, 353, (0), 7-14. |
| [78] | Maschietti, M., 2011, "High-pressure gas–liquid equilibrium of the system carbon dioxide–β-caryophyllene at 50 and 70 °C", The Journal of Supercritical Fluids, 59, 8-13. |
| [79] | Alaei, J., Tajerian, M., 2003, "Equilibrium data determination for system methane and heptane", Petroleum and Coal, 45, (1-2), 41-44. |
| [80] | Guo, H., Gong, M., Dong, X., Wu, J., 2014, "A static analytical apparatus for vapour pressures and (vapour + liquid) phase equilibrium measurements with an internal stirrer and view windows", The Journal of Chemical Thermodynamics, 76, (0), 116-123. |
| [81] | Li, H., Han, M., Gao, X., Li, X., 2014, "Isobaric vapor–liquid equilibrium for binary system of cinnamaldehyde + benzaldehyde at 10, 20 and 30 kPa", Fluid Phase Equilibria, 364, (0), 62-66. |
| [82] | Delgado, P., Sanz, M. T., Beltrán, S., 2007, "Isobaric vapor–liquid equilibria for the quaternary reactive system: Ethanol + water + ethyl lactate + lactic acid at 101.33 kPa", Fluid Phase Equilibria, 255, (1), 17-23. |
| [83] | Cheng, S.-H., Yang, F.-C., Yang, Y.-H., Hu, C.-C., Chang, W.-T., 2013, "Measurements and modeling of the solubility of ergosterol in supercritical carbon dioxide", Journal of the Taiwan Institute of Chemical Engineers, 44, (1), 19-26. |
| [84] | Kopcak, U., Mohamed, R. S., 2005, "Caffeine solubility in supercritical carbon dioxide/co-solvent mixtures", The Journal of Supercritical Fluids, 34, (2), 209-214. |
| [85] | Fonseca, J., Simoes, P. C., Nunes da Ponte, M., 2003, "An apparatus for high-pressure VLE measurements using a static mixer. Results for (CO2+limonene+citral) and (CO2+limonene+linalool)", The Journal of Supercritical Fluids, 25, (1), 7-17. |
| [86] | Saldaña, M. D. A., Tomberli, B., Guigard, S. E., Goldman, S., Gray, C. G., Temelli, F., 2007, "Determination of vapor pressure and solubility correlation of phenolic compounds in supercritical CO2", The Journal of Supercritical Fluids, 40, (1), 7-19. |
| [87] | Iwai, Y., Nagano, H., Lee, G. S., Uno, M., Arai, Y., 2006, "Measurement of entrainer effects of water and ethanol on solubility of caffeine in supercritical carbon dioxide by FT-IR spectroscopy", The Journal of Supercritical Fluids, 38, (3), 312-318. |
| [88] | Pereira, P. J., Coto, B., Menduiña, C., Gomes de Azevedo, E., Nunes da Ponte, M., 2004, "High pressure phase equilibrium for δ-tocopherol + CO2", Fluid Phase Equilibria, 216, (1), 53-57. |
| [89] | Johannsen, M., Brunner, G., 1994, "Solubilities of the xanthines caffeine, theophylline and theobromine in supercritical carbon dioxide", Fluid Phase Equilibria, 95, 215-226. |
| [90] | Yeoh, H. S., Chong, G. H., Azahan, N. M., Rhaman, R. A., Choong, T. S. Y., 2013, "Solubility measurement method and mathematical modeling in supercritical fluids", Engineering Journal, 17, 67-78. |
| [91] | Yokozeki, A., Shiflett, M. B., 2011, "The solubility of CO2 and N2O in olive oil", Fluid Phase Equilibria, 305, (2), 127-131. |
| [92] | Saldaña, M. D. A., Valdivieso-Ramírez, C. S., 2015, "Pressurized fluid systems: Phytochemical production from biomass", The Journal of Supercritical Fluids, 96, 228-244. |
| [93] | Ferrentino, G., Balzan, S., Spilimbergo, S., 2012, "On-line color monitoring of solid foods during supercritical CO2 pasteurization", Journal of Food Engineering, 110, (1), 80-85. |
| [94] | Zhang, W., Kiran, E., 2003, "(p,V,T) Behaviour and miscibility of (polysulfone+THF+carbon dioxide) at high pressures", The Journal of Chemical Thermodynamics, 35, (4), 605-624. |
| [95] | Raal, J. D., Motchelaho, A. M., Perumal, Y., Courtial, X., Ramjugernath, D., 2011, "P–x data for binary systems using a novel static total pressure apparatus", Fluid Phase Equilibria, 310, (1–2), 156-165. |
| [96] | Regueira, T., Carvalho, P. J., Oliveira, M. B., Lugo, L., Coutinho, J. A. P., Fernández, J., 2013, "Experimental measurements and modeling of CO2 solubility in sunflower, castor and rapeseed oils", The Journal of Supercritical Fluids, 82, (0), 191-199. |
| [97] | De Sousa, A. R. S., Raeissi, S., Aguiar-Ricardo, A., M. Duarte, C. M., Peters, C. J., 2004, "High pressure phase behavior of the system ethane+orange peel oil", The Journal of Supercritical Fluids, 29, (1–2), 59-67. |
| [98] | Pereira Alcântara, L. A., do Nascimento, K. S., Mourão, C. A., Minim, V. P. R., Minim, L. A., 2013, "Aqueous two-phase poly(ethylene glycol)–sodium polyacrylate system for amyloglucosidase purification: Equilibrium diagrams and partitioning studies", Separation and Purification Technology, 118, 888-894. |
| [99] | Varona, S., Braeuer, A., Leipertz, A., Martín, Á., Cocero, M. J., 2013, "Lycopene solubility in mixtures of carbon dioxide and ethyl acetate", The Journal of Supercritical Fluids, 75, 6-10. |
| [100] | Benelli, P., Rosso Comim, S. R., Vladimir Oliveira, J., Pedrosa, R. C., Ferreira, S. R. S., 2014, "Phase equilibrium data of guaçatonga (Casearia sylvestris) extract + ethanol + CO2 system and encapsulation using a supercritical anti-solvent process", The Journal of Supercritical Fluids, 93, 103-111. |
| [101] | Dalmolin, I., Rigo, A. A., Corazza, M. L., Ndiaye, P. M., Meireles, M. A. A., Batista, E. A. C., Oliveira, J. V., 2014, "Phase behaviour and thermodynamic modelling for the system (grape seed oil + carbon dioxide + ethanol) at high pressures", The Journal of Chemical Thermodynamics, 68, 71-74. |
| [102] | Debien, I. C. N., Rigo, A. A., Mazutti, M. A., Oliveira, J. V., Meireles, M. A. A., 2013, "High-pressure phase equilibrium data for the l-lactic acid + (propane + ethanol) and the l-lactic acid + (carbon dioxide + ethanol) systems", The Journal of Supercritical Fluids, 79, 27-31. |
| [103] | Ndiaye, P. M., Franceschi, E., Oliveira, D., Dariva, C., Tavares, F. W., Oliveira, J. V., 2006, "Phase behavior of soybean oil, castor oil and their fatty acid ethyl esters in carbon dioxide at high pressures", The Journal of Supercritical Fluids, 37, (1), 29-37. |
| [104] | Peper, S., Haverkamp, V., Dohrn, R., 2010, "Measurement of phase equilibria of the systems CO2 + styrene and CO2 + vinyl acetate using different experimental methods", The Journal of Supercritical Fluids, 55, (2), 537-544. |
| [105] | De la Fuente, J. C., Valderrama, J. O., Bottini, S. B., del Valle, J. M., 2005, "Measurement and modeling of solubilities of capsaicin in high-pressure CO2", The Journal of Supercritical Fluids, 34, (2), 195-201. |
| [106] | García-Abarrio, S. M., Haya, L., Pardo, J. I., Urieta, J. S., Mainar, A. M., 2013, "Isobaric VLE of the mixture {(±)-linalool + ethanol}: A case study for the distillation of absolute and volatile oils", The Journal of Chemical Thermodynamics, 64, 182-186. |
| [107] | Olson, J. D., 1989, "Measurement of vapor-liquid equilibria by ebulliometry", Fluid Phase Equilibria, 52, 209-218. |
| [108] | Deiters, U. K., Scheider, G. M., 1986, "High pressure phase equilibria: experimental methods", Fluid Phase Equilibria, 29, (5), 145-160. |
| [109] | Mohammad-Taheri, M., Zarringhalam Moghaddam, A., Nazari, K., Gholipour Zanjani, N., 2013, "The role of thermal path on the accuracy of gas hydrate phase equilibrium data using isochoric method", Fluid Phase Equilibria, 338, 257-264. |
| [110] | Kao, L., Chen, C.-R., Chang, C.-M. J., 2007, "Supercritical CO2 extraction of turmerones from turmeric and high-pressure phase equilibrium of CO2 + turmerones", The Journal of Supercritical Fluids, 43, (2), 276-282. |
| [111] | Michielin, E. M. Z., Rosso, S. R., Franceschi, E., Borges, G. R., Corazza, M. L., Oliveira, J. V., Ferreira, S. R. S., 2009, "High-pressure phase equilibrium data for systems with carbon dioxide, α-humulene and trans-caryophyllene", The Journal of Chemical Thermodynamics, 41, (1), 130-137. |
| [112] | Borges, G. R., Junges, A., Franceschi, E., Corazza, F. C., Corazza, M. L., Oliveira, J. V., Dariva, C., 2007, "High-Pressure Vapor−Liquid Equilibrium Data for Systems Involving Carbon Dioxide + Organic Solvent + β-Carotene", Journal of Chemical & Engineering Data, 52, (4), 1437-1441. |
| [113] | Pereira, L., Santos, P. G. d., Scheer, A. P., Ndiaye, P. M., Corazza, M. L., 2014, "High pressure phase equilibrium measurements for binary systems CO2+1-pentanol and CO2+1-hexanol", The Journal of Supercritical Fluids, 88, 38-45. |
| [114] | Kotnik, P., Škerget, M., Knez, Ž., 2014, "Phase equilibria of free fatty acids enriched vegetable oils and carbon dioxide: Experimental data, distribution coefficients and separation factors", The Journal of Supercritical Fluids, 87, 65-72. |
| [115] | Rodrigues, J. E., Araújo, M. E., Azevedo, F. F. M., Machado, N. T., 2005, "Phase equilibrium measurements of Brazil nut (Bertholletia excelsa) oil in supercritical carbon dioxide", The Journal of Supercritical Fluids, 34, (2), 223-229. |
| [116] | Carvalho, R. N., Corazza, M. L., Cardozo-Filho, L., Meireles, M. A. A., 2006, "Phase Equilibrium for (Camphor + CO2), (Camphor + Propane), and (Camphor + CO2 + Propane)", Journal of Chemical & Engineering Data, 51, (3), 997-1000. |
| [117] | Duarte, C. M. M., Crew, M., Casimiro, T., Aguiar-Ricardo, A., Nunes da Ponte, M., 2002, "Phase equilibrium for capsaicin+water+ethanol+supercritical carbon dioxide", The Journal of Supercritical Fluids, 22, (2), 87-92. |
| [118] | Gironi, F., Maschietti, M., 2011, "High-pressure gas–liquid equilibrium of the system carbon dioxide–citral at 50 and 70 °C", The Journal of Supercritical Fluids, 57, (1), 25-30. |
| [119] | Venter, M. J., Willems, P., Kareth, S., Weidner, E., Kuipers, N. J. M., de Haan, A. B., 2007, "Phase equilibria and physical properties of CO2-saturated cocoa butter mixtures at elevated pressures", The Journal of Supercritical Fluids, 41, (2), 195-203. |
| [120] | Paviani, L. C., Chiari, M. R. S., Crespo, T. R., Cabral, F. A., "Thermodynamic Modelling of Phase Equilibrium Behavior of Curcumin-CO2-Ethanol", in III Iberoamerican Conference on Supercritical Fluids, 2013. |
| [121] | Crevatin, A., Zwahlen, A., Kikic, I., 1998, "High pressure phase equilibrium for binary system dl-α tocopherol + methanol", The Journal of Supercritical Fluids, 12, (2), 99-108. |
| [122] | Crevatin, A., Steiner, K., Kikic, I., 1999, "High pressure vapour–liquid equilibria for the mixture dl-γ-tocopherol/methanol", Fluid Phase Equilibria, 157, (1), 103-109. |
| [123] | Simões, P. C., Brunner, G., 1996, "Multicomponent phase equilibria of an extra-virgin olive oil in supercritical carbon dioxide", The Journal of Supercritical Fluids, 9, (2), 75-81. |
| [124] | Moura, L. S., Corazza, M. L., Cardozo-Filho, L., Meireles, M. A. A., 2005, "Phase Equilibrium Measurements for the System Fennel (Foeniculum vulgare) Extract + CO2", Journal of Chemical & Engineering Data, 50, (5), 1657-1661. |
| [125] | Gironi, F., Maschietti, M., 2006, "Separation of fish oils ethyl esters by means of supercritical carbon dioxide: Thermodynamic analysis and process modelling", Chemical Engineering Science, 61, (15), 5114-5126. |
| [126] | Riha, V., Brunner, G., 1999, "Phase equilibrium of fish oil ethyl esters with supercritical carbon dioxide", The Journal of Supercritical Fluids, 15, (1), 33-50. |
| [127] | de la Fuente B, J. C., Bottini, S. B., 2000, "High-pressure phase equilibria and thermodynamic modelling for the binary systems CO2+lemon oil and C2H6+lemon oil", Fluid Phase Equilibria, 175, (1–2), 45-52. |
| [128] | Franceschi, E., Grings, M. B., Frizzo, C. D., Oliveira, J. V., Dariva, C., 2004, "Phase behavior of lemon and bergamot peel oils in supercritical CO2", Fluid Phase Equilibria, 226, (0), 1-8. |
| [129] | Gutiérrez, C., Rodríguez, J. F., Gracia, I., de Lucas, A., García, M. T., 2013, "High-pressure phase equilibria of Polystyrene dissolutions in Limonene in presence of CO2", The Journal of Supercritical Fluids, 84, 211-220. |
| [130] | Florusse, L. J., Fornari, T., Bottini, S. B., Peters, C. J., 2004, "Phase behavior of carbon dioxide—low-molecular weight triglycerides binary systems: measurements and thermodynamic modeling", The Journal of Supercritical Fluids, 31, (2), 123-132. |
| [131] | Sovová, H., Stateva, R. P., Galushko, A. A., 2007, "High-pressure equilibrium of menthol + CO2", The Journal of Supercritical Fluids, 41, (1), 1-9. |
| [132] | Rebocho, S., Nunes, A. V. M., Najdanovic-Visak, V., Barreiros, S., Simões, P., Paiva, A., 2014, "High pressure vapor–liquid equilibrium for the ternary system ethanol/(±)-menthol/carbon dioxide", The Journal of Supercritical Fluids, 92, 282-287. |
| [133] | Ruivo, R., Paiva, A., Simões, P., 2004, "Phase equilibria of the ternary system methyl oleate/squalene/carbon dioxide at high pressure conditions", The Journal of Supercritical Fluids, 29, (1–2), 77-85. |
| [134] | Hong, S.-A., Kim, J.-D., Kim, J., Kang, J. W., Kang, I.-J., 2010, "Phase equilibria of palm oil, palm kernel oil, and oleic acid + supercritical carbon dioxide and modeling using Peng–Robinson EOS", Journal of Industrial and Engineering Chemistry, 16, (5), 859-865. |
| [135] | Fernández-Ronco, M. P., Gracia, I., De Lucas, A., Rodríguez, J. F., 2011, "Measurement and modeling of the high-pressure phase equilibria of CO2-Oleoresin Capsicum", The Journal of Supercritical Fluids, 57, (2), 112-119. |
| [136] | Stuart, G. R., Dariva, C., Oliveira, J. V., 2000, "High-Pressure Vapor-Liquid Equilibrium Data for CO2-Orange Peel Oil", Brazillian Journal of Chemical Engineering, 17, (2), 181-189. |
| [137] | Rosso Comim, S. R., Franceschi, E., Borges, G. R., Corazza, M. L., Vladimir Oliveira, J., Ferreira, S. R. S., 2010, "Phase equilibrium measurements and modelling of ternary system (carbon dioxide + ethanol + palmitic acid)", The Journal of Chemical Thermodynamics, 42, (3), 348-354. |
| [138] | Corrêa, F. V., Comim, S. R. R., de Cesaro, A. M., Rigo, A. A., Mazutti, M. A., Hense, H., Oliveira, J. V., 2011, "Phase equilibrium data for the ternary system (propane + chloroform + oryzanol)", The Journal of Chemical Thermodynamics, 43, (1), 34-38. |
| [139] | Ilić, L., Škerget, M., Hrnčič, M. K., Knez, Ž., 2009, "Phase behavior of sunflower oil and soybean oil in propane and sulphur hexafluoride", The Journal of Supercritical Fluids, 51, (2), 109-114. |
| [140] | Li, J., Rodrigues, M., Paiva, A., Matos, H. A., Azevedo, E. G. d., 2006, "Binary solid–liquid–gas equilibrium of the tripalmitin/CO2 and ubiquinone/CO2 systems", Fluid Phase Equilibria, 241, (1–2), 196-204. |
| [141] | Hlengwere, A., Iwarere, S. A., Naidoo, P., Raal, J. D., Ramjugernath, D., 2014, "Vapour–liquid equilibrium of propionic acid + caproic acid, isobutyric acid + caproic acid, valeric acid + caproic acid and caproic acid + enanthoic acid binary mixtures", Fluid Phase Equilibria, 375, 201-208. |
| [142] | Knez, Ž., Škerget, M., Uzunalić, A. P., 2007, "Phase equilibria of vanillins in compressed gases", The Journal of Supercritical Fluids, 43, (2), 237-248. |
| [143] | Knez, Ž., Škerget, M., 2001, "Phase equilibria of the vitamins D2, D3 and K3 in binary systems with CO2 and propane", The Journal of Supercritical Fluids, 20, (2), 131-144. |
| [144] | Chen, Y., Wang, H., Tang, Y., Zeng, J., 2012, "Ternary (liquid + liquid) equilibria for (water + 2-propanol + α-pinene, or β-pinene) mixtures at four temperatures", The Journal of Chemical Thermodynamics, 51, 144-149. |
| [145] | Toure, O., Audonnet, F., Lebert, A., Dussap, C.-G., 2015, "Development of a thermodynamic model of aqueous solution suited for foods and biological media. Part A: Prediction of activity coefficients in aqueous mixtures containing electrolytes", The Canadian Journal of Chemical Engineering, 93, (2), 443-450. |
| [146] | Benmekki, E. H., Mansoori, G. A., 1987, "Phase equilibrium calculations of highly polar systems", Fluid Phase Equilibria, 32, (2), 139-149. |
| [147] | O´Connell, J. P., Haile, J. M., Thermodynamics Fundamentals for Applications, Cambridge University Press, 2011. |
| [148] | Michelsen, M. L., Mollerup, J., Thermodynamic Models: Fundamentals and Computational Aspects, 2nd ed., Tie-Line Publications, 2007. |
| [149] | Redlich, O., Kwong, J. N. S., 1949, "On the Thermodynamics of Solutions. V. An Equation of State. Fugacities of Gaseous Solutions", Chemical Reviews, 44, (1), 233-244. |
| [150] | Soave, G., 1972, "Equilibrium constants from a modified Redlich-Kwong equation of state", Chemical Engineering Science, 27, (6), 1197-1203. |
| [151] | Peng, D.-y., Robinson, D. B., 1977, "A rigorous method for predicting the critical properties of multicomponent systems from an equation of state", AIChE Journal, 23, (2), 137-144. |

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML