| [1] | R. Martinez, P. Torres, M.A. Meneses, J.G. Figueroa, J.A. Pérez-Álvarez, M. Viuda-Martos, “Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate”, Food Chemistry, vol. 135, pp. 1520-1526, 2012. |
| [2] | A.C. Oliveira, I.B. Valentim, C.A. Silva, E.J.H. Bechara, M.P. Barros, C.M. Mano, M.O.F. Goulart, “Total phenolic content and free radical scavenging activities of methanolic extract powders of tropical fruit residues”, Food Chemistry, vol. 115, pp. 469-475, 2009. |
| [3] | J.K. Silva, C.B.B. Cazarin, T.C. Colomeu, Â.G. Batista, L.M.M. Meletti, J.A.R. Paschoal, S. Bogusz Júnior, M.F. Furlan, F.G.R. Reyes, F. Augusto, M.R. Maróstica Júnior, R. de Lima Zollner, “Antioxidant activity of aqueous extract of passion fruit (Passiflora edulis) leaves: In vitro and in vivo study”, Food Research International, vol. 53, pp. 882-890, 2013. |
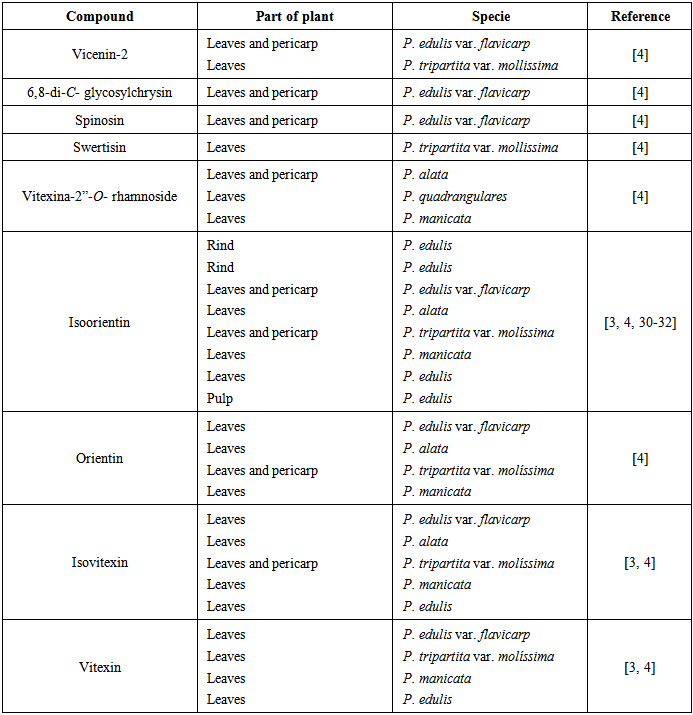
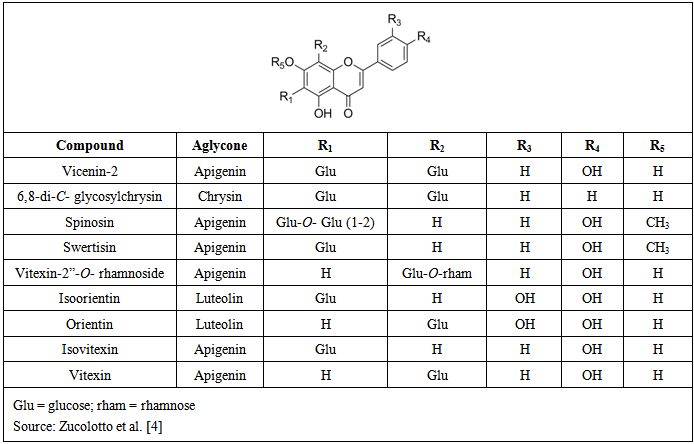
| [4] | S.M. Zucolotto, C. Fagundes, F.H. Reginatto, F.A. Ramos, L. Castellanos, C. Duque, E.P. Schenkel, “Analysis of C-glycosyl flavonoids from South American Passiflora species by HPLC-DAD and HPLC-MS”, Phytochemical Analysis, vol. 23, pp. 232-239, 2012. |
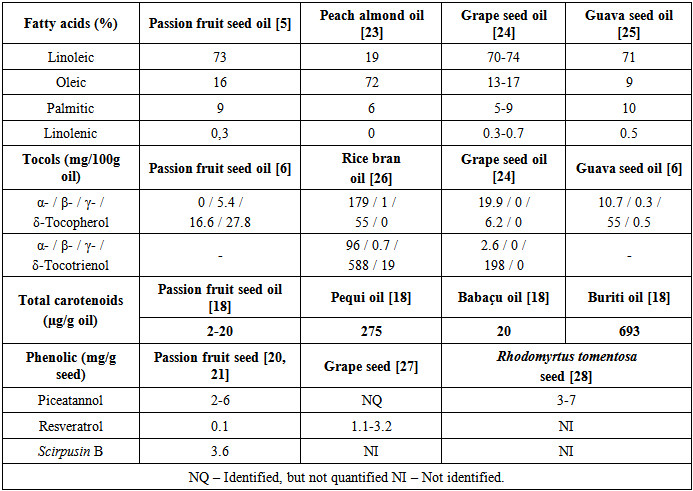
| [5] | S. Liu, F. Yang, C. Zhang, H. Ji, P. Hong, C. Deng, “Optimization of process parameters for supercritical carbon dioxide extraction of Passiflora seed oil by response surface methodology”, The Journal of Supercritical Fluids, vol. 48 pp. 9-14, 2009. |
| [6] | C.R. Malacrida, N. Jorge, “Yellow Passion Fruit Seed Oil (Passiflora edulis f. flavicarpa): Physical and Chemical Characteristics”, Brazilian archives of biology and technology, vol. 55, pp. 127-134, 2012. |
| [7] | B.S. Ferreira, C.G. de Almeida, M. Le Hyaric, V.E. de Oliveira, H.G.M. Edwards, L.F.C. de Oliveira, “Raman Spectroscopic Investigation of Carotenoids in Oils from Amazonian Products”, Spectroscopy Letters, vol. 46, pp. 122-127, 2013. |
| [8] | M.L. Zeraik, C.A.M. Pereira, V.G. Zuin, J.H. Yariwake, “Maracujá: um alimento funcional?”, Brazilian Journal of Pharmacognosy, vol. 20, pp. 459-471, 2010. |
| [9] | G.P. Gerola, N.V. Boas, J. Caetano, C.R.T. Tarley, A.C. Gonçalves, D.C. Dragunski, “Utilization of Passion Fruit Skin By-Product as Lead(II) Ion Biosorbent”, Water, Air, & Soil Pollution, vol. 224, pp. 1-11, 2013. |
| [10] | C.B.B. Cazarin, J.K.d. Silva, T.C. Colomeu, R.d.L. Zollner, M.R. Maróstica Junior, “Capacidade antioxidante e composição química da casca de maracujá (Passiflora edulis)”, Ciência Rural, vol. 44, pp. 1699-1704, 2014. |
| [11] | IBGE, Produção agricola municipal 2012 (PAM2012), Instituto Brasileiro de Geografia e Estatística, [Online]. Available:http://www.ibge.gov.br/home/estatistica/pesquisas/pesquisa_resultados.php?id_pesquisa=44, 2014. |
| [12] | C.F. Chau, Y.L. Huang, “Characterization of passion fruit seed fibres - A potential fibre source”, Food Chemistry, vol. 85, pp. 189-194, 2004. |
| [13] | J. Martínez, A.C.d. Aguiar, Extraction of Triacylglycerols and Fatty Acids Using Supercritical Fluids - Review, Current Analytical Chemistry, 10 (2014) 67-77. |
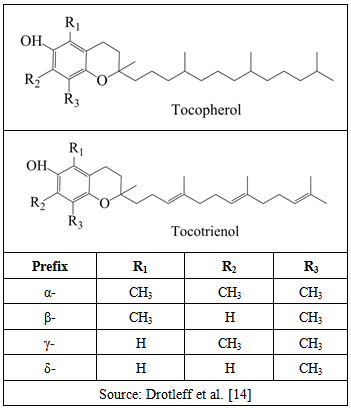
| [14] | A.M. Drotleff, C. Bohnsack, I. Schneider, A. Hahn, W. Ternes, “Human oral bioavailability and pharmacokinetics of tocotrienols from tocotrienol-rich (tocopherol-low) barley oil and palm oil formulations”, Journal of Functional Foods, vol. 7, pp. 150-160, 2014. |
| [15] | X. Zhang, Y. Shen, W. Prinyawiwatkul, J.M. King, Z. Xu, “Comparison of the activities of hydrophilic anthocyanins and lipophilic tocols in black rice bran against lipid oxidation”, Food Chemistry, vol. 141, pp. 111-116, 2013. |
| [16] | M.T. Labuschagne, N. Mkhatywaa, B. Wentzel, E. Johansson, A.V. Biljon, “Tocochromanol concentration, protein composition and baking quality of white flour of South African wheat cultivars”, Journal of Food Composition and Analysis, vol. 33, pp. 127–131, 2014. |
| [17] | S.A. Heleno, L. Barros, M.J. Sousa, A. Martins, I.C.F.R. Ferreira, “Tocopherols composition of Portuguese wild mushrooms with antioxidant capacity”, Food Chemistry, vol. 119, pp. 1443–1450, 2010. |
| [18] | B.S. Ferreira, C.G. de Almeida, L.P. Faza, A. de Almeida, C.G. Diniz, V.L. da Silva, R.M. Grazul, M. Le Hyaric, “Comparative properties of Amazonian oils obtained by different extraction methods”, Molecules, vol. 16, pp. 5875-5885, 2011. |
| [19] | E. Murillo, D. Giuffrida, D. Menchaca, P. Dugo, G. Torre, A.J. Melendez-Martinez, L. Mondello, “Native carotenoids composition of some tropical fruits”, Food Chemistry, vol. 140, pp. 825-836, 2013. |
| [20] | Y. Matsui, K. Sugiyama, M. Kamei, T. Takahashi, T. Suzuki, Y. Katagata, T. Ito, “Extract of Passion Fruit (Passiflora edulis) Seed Containing High Amounts of Piceatannol Inhibits Melanogenesis and Promotes Collagen Synthesis”, Journal of agricultural and food chemistry, vol. 58, pp. 11112–11118, 2010. |
| [21] | S. Sano, K. Sugiyama, T. Ito, Y. Katano, A. Ishihata, “Identification of the strong vasorelaxing substance scirpusin B, a dimer of piceatannol, from passion fruit (Passiflora edulis) seeds”, Journal of agricultural and food chemistry, vol. 59, pp. 6209-6213, 2011. |
| [22] | Y. Matsumoto, N. Gotoh, S. Sano, K. Sugiyama, T. Ito, Y. Abe, Y. Katano, A. Ishihata, “Effects of Scirpusin B, A polyphenol in passion fruit seeds, on the coronary circulation of the isolated perfused rat heart”, International Journal of Medical Research & Health Sciences, vol. 3, pp. 547-553, 2014. |
| [23] | N. Mezzomo, B.R. Mileo, M.T. Friedrich, J. Martinez, S.R. Ferreira, “Supercritical fluid extraction of peach (Prunus persica) almond oil: process yield and extract composition”, Bioresource Technology, vol. 101, pp. 5622-5632, 2010. |
| [24] | L. Fiori, V. Lavelli, K.S. Duba, P.S.C. Sri Harsha, H.B. Mohamed, G. Guella, “Supercritical CO2 extraction of oil from seeds of six grape cultivars: Modeling of mass transfer kinetics and evaluation of lipid profiles and tocol contents”, The Journal of Supercritical Fluids, vol. 94, pp. 71-80, 2014. |
| [25] | G. Piombo, N. Barouh, B. Barea, R. Boulanger, P. Brat, M. Pina, P. Villeneuve, “Characterization of the seed oils from kiwi (Actinidia chinensis), passion fruit (Passiflora edulis) and guava (Psidium guajava), Oilseeds and fats”, Crops and Lipids, vol. 13, pp. 195-199, 2006. |
| [26] | C.M.P. Sarmento, S.R.S. Ferreira, H. Hense, “Supercritical fluid extraction (SFE) of rice bran oil to obtain fractions enriched with tocopherols and tocotrienols”, Brazilian Journal of Chemical Engineering, vol. 23, pp. 243-249, 2006. |
| [27] | T. PUSSA, J. FLOREN, P. KULDKEPP, A. RAAL, “Survey of Grapevine Vitis vinifera Stem Polyphenols by Liquid Chromatography−Diode Array Detection−Tandem Mass Spectrometry”, Journal of Agriculture and Food Chemistry, vol. 54, pp. 7488−7494, 2006. |
| [28] | T.N.H. Lai, C.M. André, R. Chirinos, T.B.T. Nguyen, Y. Larondelle, H. Rogez, “Optimisation of extraction of piceatannol from Rhodomyrtus tomentosa seeds using response surface methodology”, Separation and Purification Technology, vol. 134, pp. 139-146, 2014. |
| [29] | F.L. Oliveira, M.R.F. Nascimento, S.V. Borges, P.C.N. Ribeiro, V.R. Ruback, “Aproveitamento alternativo da casca do maracujá-amarelo (Passiflora edulis f. flavicarpa) para produção de doce em calda”, Ciência e Tecnologia de Alimentos, vol. 22, pp. 259-262, 2002. |
| [30] | M.L. Zeraik, J.H. Yariwake, Analysis of passion fruit rinds (Passiflora edulis): isoorientin quantification by hptlc and evaluation of antioxidant (radical scavenging) capacity, Química Nova, 35 (2012) 541-545. |
| [31] | M.L. Zeraik, J.H. Yariwake, “Quantification of isoorientin and total flavonoids in Passiflora edulis fruit pulp by HPLC-UV/DAD”, Microchemical Journal, vol. 96, pp. 86-91, 2010. |
| [32] | M.L. Zeraik, D. Serteyn, G. Deby-Dupont, J.N. Wauters, M. Tits, J.H. Yariwake, L. Angenot, T. Franck, “Evaluation of the antioxidant activity of passion fruit (Passiflora edulis and Passiflora alata) extracts on stimulated neutrophils and myeloperoxidase activity assays”, Food Chemistry, vol. 128, pp. 259-265, 2011. |
| [33] | I. Ignat, I. Volf, V.I. Popa, “A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables”, Food Chemistry, vol. 126, pp. 1821-1835. 2011. |
| [34] | C.D. Stalikas, “Extraction, separation, and detection methods for phenolic acids and flavonoids”, Journal of separation science, vol. 30, pp. 3268-3295, 2007. |
| [35] | J.K. Silva, C.B.B. Cazarin, Â.G. Batista, M. Maróstica, “Effects of passion fruit (Passiflora edulis) byproduct intake in antioxidant status of Wistar rats tissues”, LWT - Food Science and Technology, vol. 59, pp. 1213-1219, 2014. |
| [36] | R. Tsao, R. Yang, “Optimization of a new mobile phase to know the complex and real polyphenolic composition: towards a total phenolic index using high-performance liquid chromatography”, Journal of Chromatography A, vol. 1018, pp. 29-40, 2003. |
| [37] | A.S. Grandison, M.J. Lewis, Separation processes - an overview, in: A.S. Grandison, M.J. Lewis (Eds.) Separation Processes in the food and Biotechnology Industies, Cambrige, United Kingdom: Woodhead Publishing Limited, pp. 1-15, 1996. |
| [38] | D.T. Santos, R.N. Cavalcanti, M.A. Rostagno, C.L. Queiroga, M.N. Eberlin, M.A.A. Meireles, “Extraction of Polyphenols and Anthocyanins from the Jambul (Syzygium cumini) Fruit Peels”, Food and Public Health, vol. 3 pp. 12-20, 2013. |
| [39] | H. Wijngaard, M.B. Hossain, D.K. Rai, N. Brunton, “Techniques to extract bioactive compounds from food by-products of plant origin”, Food Research International, vol. 46, pp. 505-513, 2012. |
| [40] | A.M. Farías-Campomanes, C.N. Horita, M.A.R. Pollonio, M.A.A. Meireles, “Allicin-Rich Extract Obtained from Garlic by Pressurized Liquid Extraction: Quantitative Determination of Allicin in Garlic Samples”, Food and Public Health, vol. 4, pp. 272-278, 2014. |
| [41] | P. Santos, A.C. Aguiar, G.F. Barbero, C.A. Rezende, J. Martinez, “Supercritical carbon dioxide extraction of capsaicinoids from malagueta pepper (Capsicum frutescens L.) assisted by ultrasound”, Ultrason Sonochemistry, vol. 22, pp. 78-88, 2015. |
| [42] | N. Babovic, S. Djilas, M. Jadranin, V. Vajs, J. Ivanovic, S. Petrovic, I. Zizovic, “Supercritical carbon dioxide extraction of antioxidant fractions from selected Lamiaceae herbs and their antioxidant capacity”, Innovative Food Science & Emerging Technologies, vol. 11, pp. 98-107, 2010. |
| [43] | G. Liu, X. Xu, Y. Gong, L. He, Y. Gao, “Effects of supercritical CO2 extraction parameters on chemical composition and free radical-scavenging activity of pomegranate (Punica granatum L.) seed oil”, Food and Bioproducts Processing, vol. 90, pp. 573-578, 2012. |
| [44] | I.M. Prado, G.H.C. Prado, J.M. Prado, M.A.A. Meireles, “Supercritical CO2 and low-pressure solvent extraction of mango (Mangifera indica) leaves: Global yield, extraction kinetics, chemical composition and cost of manufacturing”, Food and Bioproducts Processing, vol. 91, pp. 656-664, 2013. |
| [45] | G. Brunner, Gas extraction: an introduction to fundamentals of supercritical fluids and the application to separation processes, Darmstadt, Germany: Springer, 1994. |
| [46] | G. Brunner, “Supercritical fluids: technology and application to food processing”, Journal of Food Engineering, vol. 67, pp. 21-33, 2005. |
| [47] | J.R. Williams, T. Clifford, Supercritical fluid methods and protocols, Darmstadt, Germany: Springer, 2000. |
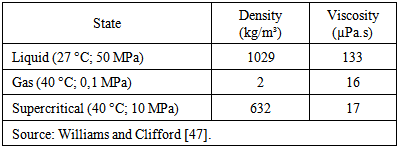
| [48] | H. Sovová, “Rate of the vegetable oil extraction with supercritical CO2 – I . Modelling of extraction curves”, Chemical Engineering Science, vol. 49, pp. 409-414, 1994. |
| [49] | O.N. Ciftci, J. Calderon, F. Temelli, “Supercritical carbon dioxide extraction of corn distiller's dried grains with solubles: experiments and mathematical modeling”, Journal of Agriculture and Food Chemistry, v. 60, pp. 12482-90, 2012. |
| [50] | G. Liu, X. Xu, Y. Gong, L. He, Y. Gao, “Effects of supercritical CO2 extraction parameters on chemical composition and free radical-scavenging activity of pomegranate (Punica granatum L.) seed oil”, Food and Bioproducts Processing, vol. 90, n. 3, pp. 573-578, 2012. |
| [51] | C.M.P. Sarmento, S.R.S. Ferreira, H. Hense, “Supercritical fluid extraction (SFE) of rice bran oil to obtain fractions enriched with tocopherols and tocotrienols”, Brazilian Journal of Chemical Engineering, vol. 23, n. 2, pp. 243-249, 2006. |
| [52] | T. Fornari, G. Vicente, E. Vazquez, M.R. Garcia-Risco, G. Reglero, “Isolation of essential oil from different plants and herbs by supercritical fluid extraction”, Journal of Chromatography A, vol. 1250, pp. 34-48, 2012. |
| [53] | I.C.N. Debien, M.A.A. Meireles, “Supercritical Fluid Extraction of Beta-ecdysone from Brazilian Ginseng (Pfaffia glomerata) Roots”, Food and Public Health, vol. 4, pp. 67-73, 2014. |
| [54] | Z.Y. JU, L.R. HOWARD, “Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin”, Journal of agricultural and food chemistry, vol. 51, pp. 5207−5213, 2003. |
| [55] | D.T. Santos, P.C. Veggi, M.A.A. Meireles, “Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins”, Journal of Food Engineering, vol. 108, pp. 444-452, 2012. |
| [56] | A. Mustafa, C. Turner, “Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review”, Analytica Chimica Acta, vol. 703, pp. 8-18, 2011. |
| [57] | H. Wijngaard, N. Brunton, “The optimization of extraction of antioxidants from apple pomace by pressurized liquids”, Journal of agricultural and food chemistry, vol. 57, pp. 10625-10631, 2009. |
| [58] | F. Temelli, O.N. Ciftci, “Developing an integrated supercritical fluid biorefinery for the processing of grains”, The Journal of Supercritical Fluids, vol. 96, pp. 77-85, 2015. |
| [59] | J. Viganó, A.P.d.F. Machado, J. Martínez, “Sub- and supercritical fluid technology applied to food waste processing”, The Journal of Supercritical Fluids, vol. 96, pp. 272–286, 2015. |
| [60] | J.T. Paula, L.C. Paviani, M.A. Foglio, I.M.O. Sousa, F.A. Cabral, “Extraction of anthocyanins from Arrabidaea chica in fixed bed using CO2 and CO2/ethanol/water mixtures as solvents”, The Journal of Supercritical Fluids, vol. 81, pp. 33-41, 2013. |
| [61] | A.T. Serra, I.J. Seabra, M.E.M. Braga, M.R. Bronze, H.C. de Sousa, C.M.M. Duarte, “Processing cherries (Prunus avium) using supercritical fluid technology. Part 1: Recovery of extract fractions rich in bioactive compounds”, The Journal of Supercritical Fluids, vol. 55, pp. 184-191, 2010. |
| [62] | T.T. Garmus, L.C. Paviani, C.L. Queiroga, P.M. Magalhães, F.A. Cabral, “Extraction of phenolic compounds from pitanga (Eugenia uniflora L.) leaves by sequential extraction in fixed bed extractor using supercritical CO2, ethanol and water as solvents”, The Journal of Supercritical Fluids, vol. 86, pp. 4-14, 2014. |
| [63] | T.T. Garmus, L.C. Paviani, C.L. Queiroga, F.A. Cabral, “Extraction of phenolic compounds from pepper-rosmarin (Lippia sidoides Cham.) leaves by sequential extraction in fixed bed extractor using supercritical CO2, ethanol and water as solvents”, The Journal of Supercritical Fluids, vol. 99, pp. 68-75, 2015. |
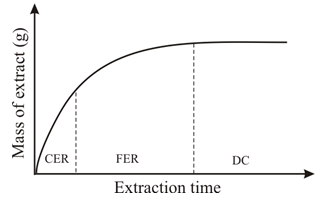
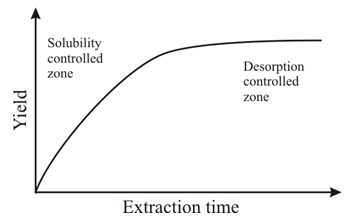
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML