-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2015; 5(4): 108-113
doi:10.5923/j.fph.20150504.02
Effect of Soaking and Germination on Proximate Composition, Mineral Bioavailability and Functional Properties of Chickpea Flour
Beruk Berhanu Desalegn
Food Science and Post-harvest Technology Program Coordinator, School of Nutrition, Food Science and Technology, Hawassa University, Southern Ethiopia
Correspondence to: Beruk Berhanu Desalegn , Food Science and Post-harvest Technology Program Coordinator, School of Nutrition, Food Science and Technology, Hawassa University, Southern Ethiopia.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The objective of this study was to assess the effect of soaking and germination on proximate composition, mineral bioavailability (Fe, Zn and Ca) and functional properties (bulk density, water absorption and oil absorption capacity) of chickpea. Chickpea seeds were soaked for 24hrs or soaked and germinated subsequently each for 24hrs. Standard methods and analytical procedures were used to analyze proximate composition, minerals, anti-nutrients and functional properties. The result revealed that, soaking and germination significantly (p<0.05) decreased protein and fiber contents, but increased carbohydrate contents and energy. Likewise, soaking and germination statistically (p<0.05) increased bioavailability of Fe and Ca. As conclusion, soaking and germination can be used for improvement of nutrient bioavailability and nutrient density.
Keywords: Soaking, Germination, Kabuli chickpea, Bioavailability
Cite this paper: Beruk Berhanu Desalegn , Effect of Soaking and Germination on Proximate Composition, Mineral Bioavailability and Functional Properties of Chickpea Flour, Food and Public Health, Vol. 5 No. 4, 2015, pp. 108-113. doi: 10.5923/j.fph.20150504.02.
Article Outline
1. Introduction
- Legumes play an important role in agriculture sector and contribute a lot for diet and also a major source of important nutrients for many people both in developed and developing countries [1, 2]. Among the different legumes, chickpea (Cicer arietinum L.) is categorized in Fabaceae (Leguminosae) family, one of the oldest and most widely consumed legumes in the world and it is a staple food crop particularly in tropical and subtropical areas [3, 4]. There are two main commercially available types of chickpea grown in the world: the desi and the kabuli. Kabuli chickpea (Garbanzo beans) is larger than desi chickpea, has a thin light-colored seed coat and is normally grown in temperate regions of the world [5]. The kabuli chickpea have normally larger seeds, better water uptake properties, shorter cooking time, lower crude fiber and higher caloric value than desi type [6]. Ethiopia is one of the major producers of chickpea and ranked eighth worldwide in 2005 and serves as a multi-purpose crop [7, 8]. Of the different purposes chickpea is giving, it can improve the nutritional status and human health due to its medicinal purpose and being a major source of protein, fiber, complex carbohydrates, vitamins, and minerals especially for those who cannot afford livestock products [9, 10, 11]. In Ethiopia, the use of chickpea grains for human food has long history and used in different forms as green vegetable (green immature seed), ‘Kollo’ (soaked and roasted), ‘nifro’ (boiled seeds) and ‘wot’ (sauces) made up of ‘shiro’ (powdered seeds) or blended with cereals and/or legumes for preparing of infant and young children foods using traditional food processing techniques like soaking, germination, fermentation, boiling, roasting etc. For preparation of infant and young children at certain community, bioavailability of macro and micro-nutrients like protein, Fe, Zn etc are critical beside sensory acceptability, cost for purchasing and processing and preparing using local food items [12]. Chickpea is known with having phytate and tannin which will bind with minerals like Fe, Zn etc and protein respectively subsequently it will decrease the bioavailability and digestibility unless appropriate and affordable processing techniques are implemented. Soaking is often used as a pretreatment to facilitate processing of legume grains and cereal seeds. In household situations cereals and legumes are typically soaked in water at room temperatures overnight [13]. Germination is a food processing method by which the quality of a cereal can be improved for both digestibility and physiological function, particularly through the breakdown of certain anti- nutrients, such as phytate, tannin and protease inhibitors [14]. During germination, enzymatic activity and bioactive compounds increased within the seed [15, 16]. Therefore, the objective of this study was to assess the effect of soaking and germination on proximate composition, mineral bioavailability (Fe, Zn and Ca) and functional properties (bulk density, water absorption and oil absorption capacity) of chickpea flours.
2. Materials and Methods
2.1. Sample Collection and Preparation
- Chickpea ((Cicer arietinum L.) kabulli variety) was collected from Debre-zeit agricultural research centre. The chickpea grains which was used in this study passed through winnowing and hand sorting in order to remove stones, dust materials, glumes, stalks, and broken, undersized and immature grains. The grains was then divided in to three as control, soaked for one day or soaked in one day in clean tap water with repeatedly changing the water, drained and germinated for a day at room temperature. Following these, the three chickpea samples (control, soaked and germinated) were sun dried, milled, sieved at 1mm (Axel Kistner, London, England) separately and each chickpea flours packed using high density polyethylene bag (HDPP) respectively. The average daily temperature during sun drying was in between 22 to 24.5°C.
2.2. Experimental Design
- Completely randomized design was used in this experiment and the effect of processing (soaking or soaking and germination) on proximate composition, minerals (Fe, Zn and Ca), anti-nutrients (phytate and condensed tannin), bioavailability of minerals (phytate: Fe molar ratio, phytate: Zn molar ratio phytate: Ca molar ratio) and functional properties (BD, WAC and OAC) of chickpea flours were studied.
2.3. Proximate Composition, Mineral Bioavailability and Functional Properties Analysis
- The proximate compositions (moisture content, crude protein, crude fat and total ash were analyzed using American association of analytical chemists (AOAC) standard methods [17]. The total carbohydrate contents were obtained by subtracting protein, ash, fat and moisture contents from 100 where as gross energy’s of the flours were calculated by using the Atwater’s conversion factor, 16.7 kJ/g for protein and carbohydrate and 37.4 kJ/g for fat. Fe, Zn and Ca contents of the flour were determined by standard procedure using an Atomic Absorption Spectrophotometer [18]. Anti-nutrients like condensed tannin and phytate contents were analyzed using the procedure followed by [16]. The bioavailability of minerals (Fe, Zn and Ca) was expressed as molar ratio of phytate and mineral (Fe or Zn or Ca). The mole of phytic acid was calculated as measured value of phytic acid divided by molecular weight of phytic acid (240) whereas the mole of mineral (Fe or Zn or Ca) was calculated as measured value of the mineral divided by individual mineral molecular weight (Fe: 56, Zn: 65, Ca:40) [19]. The functional properties like bulk density, water absorption capacity and oil absorption capacities of complementary flours were also determined by [20, 21, 22].
2.4. Statistical Analysis
- The data which were obtained in this experiment subjected to one way analysis of variance (ANOVA) using SAS 9.1 software. The mean separation values were determined using Fischer LSD test and significant differences were defined at p<0.05. The results were presented as mean ± standard deviation.
3. Results
3.1. Proximate Composition of Chickpea Flours
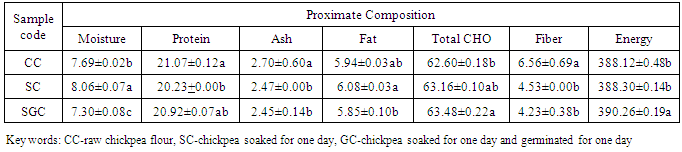
- The moisture contents of chickpea flours were in the range of 7.30 to 8.06%, but all chickpea flours (control, soaked and germinated) were significantly different (p<0.05) from each other. Soaking of chickpea showed significantly higher value (8.06%), but the lowest moisture content was exhibited in germinated chickpea. The protein contents of chickpea which was used as control had highest value than both which were soaked significantly (p<0.05) and germinated chickpea. There were significance difference (p<0.05) between control and soaked or germinated chickpea in both ash and fiber contents of chickpea fours. Higher content of ash and fiber contents were shown in the control chickpea flour. The carbohydrate and energy contents of the chickpea flours were in the range of 62.60 to 63.48% and 388.12 to 390.26kcal (Table 1).
|
3.2. Minerals and Anti-nutrients of Chickpea Flours
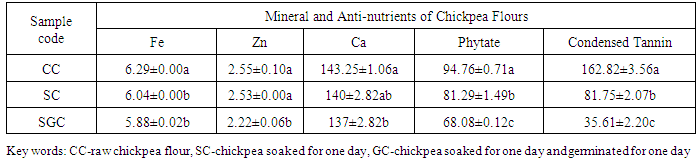
- According to Table 2, the iron contents of chickpea flours were significantly reduced when soaking and germinated applied, but there were no significant difference (p<0.05) between soaked and germinated chickpea. Though the amount of zinc was decreased after soaking taken palce, it was not statistically different (p<0.05) from the control. The lowest value of zinc was exhibited in germinated chickpea among the three. Likewise, the amount of iron, calcium, phytate and condensed tannin were lowest in chickpea which were germinated for 24hrs, but on the opposite the highest value of all minerals and anti-nutrients were shown on control. Both phytate and condensed tannin of chickpea were significantly reduced by soaking and germination (Table 2).
|
3.3. Minerals Bioavailability of Chickpea Flours
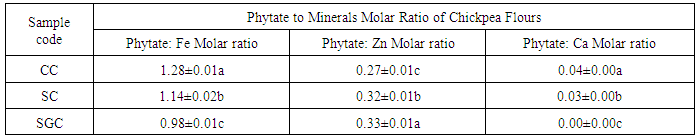
- The molar ratio of phytate to iron of control, soaked and germinated chickpea were in the range of 0.98 to 1.28, but germination significantly (p<0.05) reduced the phytate to iron ratio and followed by soaking. Similarly, phytate to calcium molar ratio decreased as soaking and germination treatments applied on chickpea. Germinated chickpea had highest phytate to zinc ratio (0.33), but the lowest was scored on control chickpea (Table 3).
|
3.4. Functional Properties of Chickpea Flours
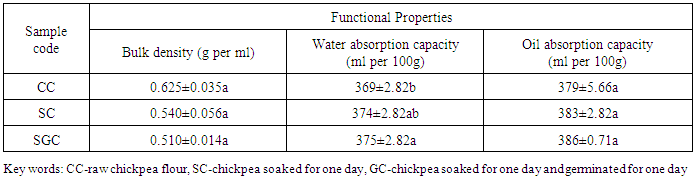
- According to Table 4, the bulk density and oil absorption capacity of chickpea flours were not significantly different (p<0.05), even though highest value of bulk density and oil absorption capacity were recorded on control and germinated chickpea. The water absorption capacity of chickpea increased as soaking and germination used, but significant decrement (p<0.05) was observed between control and germinated chickpea (Table 4).
|
4. Discussion
- Moisture content is critical for predicting the shelf life of food products. In this study, the moisture contents of chickpea flours were found to be between 7.30 and 8.06% which is appreciable for inactivation of microbial growth [23]. The finding is lower than study on African yam bean. This might be due to shorter soaking and germination time applied in this study (24hrs) than study on African yam bean and which were in the range of 24 to 48hrs for soaking and 24 to 96hrs for germination [24], but it was higher than cereal-legume composite weaning food (4.2 to 4.3%). This could be due to the efficiency of drying technique coupled with extended drying time in the later study [25]. In the present study, the amount of moisture content was increased from 7.69% to 8.06% due to soaking. Similarly, the amount of moisture contents increased from 9.75 to 10.50% in mung bean seeds [26]. In this study, germination significantly (p<0.05) decreased the moisture contents. This might be utilization of water for metabolic activities initiated by soaking. Protein contents of chickpea in this study were between 20.23 and 21.07%. The findings are lower than protein contents of mung bean (27 to 30%) and higher than chickpea’s in previous study (13.823 to 16.735%) [26, 27]. These differences might be due to mung bean is higher in protein content and the chickpea growing condition and soil types differences respectively. Protein contents in the present study showed decrement when soaked from control (21.07 to 20.23%) and subsequently increased slightly from soaked one after germination (20.23 to 20.96%). Similar fashions were followed in soaked and germinated field bean and mung bean [26, 28]. Germination decreased protein contents of chickpea from 21.23 to 20.96%. Similarly, the protein contents of legumes (kidney bean, mung bean, soya bean and peanut) and rice were decreased after germination taken place [29]. The ash contents of chickpea flours were between 2.45 to 2.70% in the present study. Comparable results were recorded in previous study on chickpea (2.30 to 2.67%) [27]. Soaking and germination of seeds significantly (p<0.05) reduced the ash contents of flours. Similar trend were observed in soaked legumes (Cejanus cajan, Labla purpureaus and Vignia ungulicalata) and germinated chickpea [27, 30]. Fat content of chickpea flours in this study showed increment due to soaking and decrement by germination processing. Similar fashion were seen in soaked legumes (Cejanus cajan, Labla purpureaus and Vignia ungulicalata) and germinated mung bean [26, 30]. The decrease due to germination could be attributed to their use as an energy source to start germination [26]. Fiber content of chickpea flours were in the range of 4.23 to 6.56% and reduced significantly (p<0.05) by soaking and germination. These might be due to degradation of fiber in to simpler sugars by initiated endogenous enzymes. This observation is in agreement with the previous studies in chickpea, mungbean, kidney bean and quality protein maize based complementary food [23, 26, 27, 29]. The carbohydrate and energy contents of chickpea flours were found to be in the range of 62.60 to 63.48% and 388.12 to 390.26kcal respectively. Comparable energy value were seen in quality protein based complementary food (388.12 to 390.26kcal) and maize based complementary diet (377.67 to 396.87kcal) [23, 31]. Likewise comparable carbohydrate were recorded in maize based complementary diet (62.94 to 66.56%) [31]. Iron, zinc and calcium contents of chickpea were in between 5.88 and 6.29mg, 2.22 and 2.55mg and 137.82 and 143.25mg respectively. Soaking and germination reduced all minerals of chickpea flours. These might be due to leaching out of minerals in to soaking water. Similar reduction of minerals (Fe, Zn and Ca) were observed in soaked lentil varieties, soaked and germinated Vigna unguiculata for poultry feed, germinated chickpea and quality protein maize based complementary food [23, 27, 30, 32]. The phytate and tannin contents of raw, soaked and germinated chickpea flours were found to be between 68.08 to 94.76mg and 35.61 to 162.82mg per 100g. Both the anti-nutrients (phytate and tannin) were significantly reduced (p<0.05) after soaking and germination processes. These might be due to increase in endogenous phytase enzyme activity and leaching of soluble tannin compounds during soaking and was further reduced after germination. The phytate: Fe Molar ratio, phytate: Zn Molar ratio and phytate: Ca Molar ratios of chickpea flours were in the range from 0.98 to 1.28, 0.27 to 0.33 and 0.00 to 0.04 respectively. The molar ratio of phytate to Zn and Ca were below the recommended maximum value 15 and 0.24 in all flours and indicated their better bioavailability for absorption, but only germinated chickpea flour had phytate to Fe ratio in the recommended range value (<1) [33, 34, 35]. Functional properties like bulk density, water absorption capacity and oil absorption capacity of food are critical to determine the application technology, its use and very important for the appropriateness of the food especially for children [23, 36]. Bulk density is one of the parameter that helps to decide the packaging material. The bulk densities of control, soaked or germinated chickpea were in the range of 0.510 to 0.625mg per ml and lower than the value of velvet bean flour (0.94 to 1.25mg per ml) [37]. Comparable result was observed in pigeon pea (0.68g per cm3) [47]. The lower bulk density value in this study would give an advantage in the formulation of complementary foods [37]. Even though the bulk density of chickpea was decreased while soaking and germination applied, it was not statistically significant (p<0.05). Similar trend was observed in quality protein maize based complementary food, millet flours for porridge production and maize grains [23, 38, 39]. Water absorption capacity of food product is an index of the maximum amount of water the product absorbs and retains and it is important to soften and increase digestibility [40]. Water absorption of chickpea flours were found to be within a range of 369 and 375ml per 100g. It was higher than water absorption capacity (129.23 to 146.80ml per 100g and 131.50 to 147.50ml per 100g) of maize based complementary foods [23, 41]. It might be due to higher amount of protein contents in the present study. This result is supported by study on soya bean-maize flour blended cookies and soya: plantain flour [48, 49]. Soaking and germination of chickpea increased water absorption from control (369 ml per 100g) to 374 and 375 ml per 100g respectively. Likewise, increased water absorption was observed in malted maize: defatted sesame blended flour and germinated lotus seed [42, 46]. Oil absorption capacity is important for nutrient and energy density of food products especially for infant and young children. In addition, the high oil absorption capacity also makes the flours suitable in facilitating enhancement in flavor and mouth feel when used in food preparations [37]. The oil absorption capacity of chickpea flours were in the range of 379 and 386 ml per 100g. This result is higher than raw (129ml per 100g), soaked in NaHCO3 and citric acid (121 and 144ml per 100g respectively) and germinated cowpea (124ml per 100g) [43]. There were no significant difference (p<0.05) between control, soaked and germinated chickpea, but it was increased in germinated seed. Similar fashion was observed in extruded millet and soya bean blended flour [44].
5. Conclusions
- Soaking and germination increased carbohydrate and energy, but it decreased anti-nutrients content (phytate and condensed tannin) and improved bioavailability of minerals (Ca and Fe) and protein digestibility. They also increased WAC and OAC, but decreased BD. Generally, soaking and germination of legumes can be used for improvement of nutrient bioavailability and nutrient density so that it is appropriate technique for production of infant and young children foods.
ACKNOWLEDGMENTS
- The authors would like to thank Hawassa University, NORAD project for financing this research work. The authors would like also to thank Hawassa University, School of Nutrition, Food Science and Technology and Ethiopian Health and Nutrition Research Institute for carrying out this experiment.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML