-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2015; 5(2): 38-46
doi:10.5923/j.fph.20150502.02
Novel Extraction Method to Produce Active Solutions from Plant Materials
R. Abel C. Torres, Diego T. Santos, M. Angela A. Meireles
LASEFI/DEA/FEA (School of Food Engineering)/UNICAMP (University of Campinas), Cidade Universitária "Zeferino Vaz", R. Monteiro Lobato, Campinas, SP, Brazil
Correspondence to: M. Angela A. Meireles, LASEFI/DEA/FEA (School of Food Engineering)/UNICAMP (University of Campinas), Cidade Universitária "Zeferino Vaz", R. Monteiro Lobato, Campinas, SP, Brazil.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
A novel selective extraction method to produce active solutions with from plant materials was developed. Named by our research group as High Turbulence Extraction Assisted by Ultrasound (HTEAU) the feasibility of this process was demonstrated using semi-defatted annatto seeds (Bixa orellana L.) as a model plant material and ethanol as extracting solvent. HTEAU process combines the use of two types of commercial equipments and technologies. The first is Ultra-turrax® rotor-stator technology, which produces high turbulence in the plant material bed by high extracting solvent circulation flow rate (until 2000 cm3/min) and the second is ultrasound technology, which is recognized to improve the extraction rate by the increasing the mass transfer and possible rupture of cell wall due the formation of microcavities. These equipments were coupled and put into operation at its maximum power of operability and the values for these parameters were determined through simultaneous optimization of oils, phenols, bixin recoveries. The effects of extraction method and solvent mass to feed mass ratio (S/F) on oils, phenols, bixin recoveries were evaluated by analyses of variance (ANOVA), demonstrating that the coupling of ultrasound probe into the Ultra-turrax® equipment statistically promotes the selective extraction of total phenols and bixin.
Keywords: Ultra-turrax, Ultrasound, Active solution,Bixa orellana L, Bixin extraction
Cite this paper: R. Abel C. Torres, Diego T. Santos, M. Angela A. Meireles, Novel Extraction Method to Produce Active Solutions from Plant Materials, Food and Public Health, Vol. 5 No. 2, 2015, pp. 38-46. doi: 10.5923/j.fph.20150502.02.
Article Outline
1. Introduction
- A wide array of solid–liquid extraction techniques is widely used for the extraction of natural products from plant solid matrixes. Solid–liquid extraction techniques has been divided into traditional and recent method categories in [1, 2]. Examples of traditional methods include maceration, leaching, and Soxhlet extraction. These techniques have been used for sometime; however, they are time-consuming and require large quantities of solvents. There is an increasing demand for new extraction techniques with shortened extraction time, reduced solvent consumption, and increased pollution prevention. Recent extraction methods such as Ultrasound Assisted Extraction (UAE), Microwave Assisted Extraction (MAE), Pressurized Liquid Extraction (PLE), Accelerated Solvent Extraction (ASE), and Supercritical Fluid Extraction (SFE) are fast and efficient for extracting chemicals from solid plant matrixes. These techniques have the possibility of working at elevated temperatures and/or pressures, greatly decreasing the time of extraction. Annatto is obtained from the outer layer of the seeds of the tropical tree Bixa orellana L. This tree is native to tropical South America; however, it is also cultivated in many countries in Central America (Mexico, Guatemala and Caribbean), Africa (Kenya, Tanzania, Côte d’Ivoire, Angola) and South Asia (India, Sri Lanka, Philippines, Vietnam). The main pigments of annatto seeds are bixin and norbixin, where bixin is the major pigment accounting for 80% of the total carotenoid content present contained in the resinous coating surrounding the seed itself. Its colour can vary in tone between yellow and red. Specifically, the major pigment present is cis-bixin; also present as minor constituents are trans-bixin, cis-norbixin and trans-norbixin.The current work is motivated by the investigation of a 2 phase approach towards extracting, fractionating, and forming particles of cis-bixin derived from Annatto seeds. The first phase is focused on the efficient production of an active solution containing components of annatto using methods consistent with large-scale industrial food processing. The second phase is focused on the fractionation and particle formation of cis-bixin using Supercritical Antisolvent Fractionation. This paper will focus on the practical issues involved in the extraction methods used for the first phase in producing active solutions with the broad range of solid–liquid extraction methods in current use. In this study the High Turbulence Extraction Assisted by Ultrasound (HTEAU) device and procedures are described in detail and successfully developed using semi-defatted annatto seeds (Bixa orellana L.) as plant material and ethanol as extracting solvent as a model case.
1.1. Overview of Extraction Methods
- The extraction of natural products from plants has likely existed since the time when humans first learned to created fire. Over the centuries, we have carried out solid-liquid extraction by brewing just about every common plant leaf, fruit, or root. In the process, we have extracted a number of compounds; many of them used for medicinal purposes.More recently, surveys of traditional and “recent” methods for the extraction of natural products have been published [1, 2]. The traditional methods cited typically include maceration [3], leaching [4-6], and Soxhlet extraction [7, 8]. More recent methods include Ultrasound Assisted Extraction (UAE) [9, 3, 10], Microwave Assisted Extraction (MAE) [3, 11], Pressurized Liquid Extraction [4, 12], Accelerated Solvent Extraction (ASE), Supercritical Fluid Extraction (SFE) [13], and certainly others can and will be devised. As suggested by Wang [1] all these forms of SLE may be generalized into a fundamental methodology of the extraction process.In its fundamental form, the SLE process consists of a reservoir of fluid solvent in contact with a solid matrix containing solute(s). The process is then typically accelerated (with regard to processing time) or otherwise optimized (e.g. for yield) using various techniques. For instance, such techniques can be (1) pack the solid matrix within a solvent permeable membrane to facilitate or eliminate filtration of the final solution, (2) circulation, recirculation, or reflux of solvent to minimize the concentration of solute in direct contact with the solid matrix aiding mass transfer kinetics, (3) elevation of temperature to increase diffusion of solute and decrease the viscosity of the solution, (4) application of vacuum or pressure to tailor the extraction process through manipulation of the solvent phase state as used in SFE [13], (5) application of sonication to introduce cavitation formation and collapse processes at the solid matrix surface [14, 15, 16] and (6) application of microwave fields to rapidly and uniformly heat polar/ionic solutions [17] and/or heat polar solutes within the solid matrix increasing intra-solid diffusion and potentially inducing cellular rupture within the solid matrix [18], however the process of superheating [19] must be considered when extracting natural products.Therefore, the practical concerns for the ultimate extraction method and these techniques include the matrix characteristics and solid particle size, solvent choice, total solvent used and solid-liquid ratio, temperature and pressure, extraction time, solvent circulation, ultrasound power and frequency, and microwave power and frequency. These concerns are frequently addressed – and their parameters tailored – by experimental study using a design of experiments that statistically model the response of pertinent objectives against a factorized combination of the above items, as in [4, 10] for example. Investigations applying all these techniques simultaneously into a single experimental investigation are more recently appearing in the literature [20].
1.2. Obtaining Active Compounds from Annatto Seeds
- Relevant methods and techniques used for the extraction of natural materials that have been published are gathered in the following. The sample of work reviewed encompasses the methods and techniques in the previous section and includes a number of studies related to the extraction of bixin from annatto.Bixin was extracted from annatto seed in [6] (Table 1) by submerging the seeds in acetone and by combined extractions using sodium hydroxide and soybean oil in ambient daylight and dark conditions. The percent yield from leaching 5 g of seeds in 20 cm3 of acetone in daylight was 70.7% after 60 min. The total bixin content within in the seeds was measured in determining the percent yield, but this total bixin content was not reported. However, assuming a typical bixin content of 1.0 to 4.9%, [4, 10, 8], the yield would be 18 to 35 g/kg.
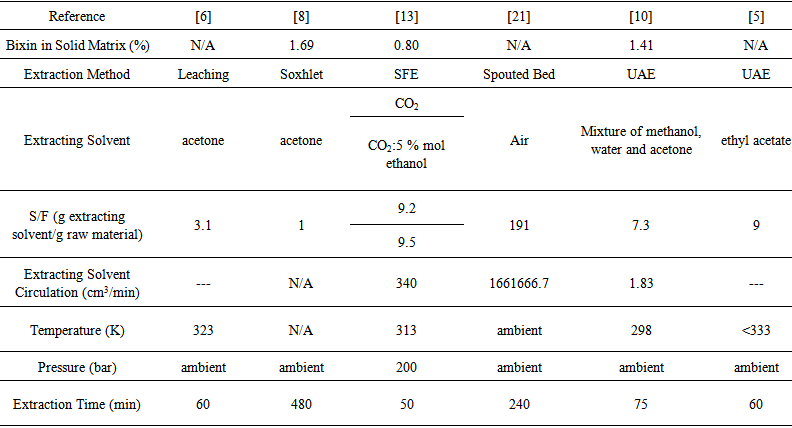 | Table 1. Obtaining of bixin-rich extract using different extraction methods from annatto seeds |
2. Materials and methods
2.1. Plant Material Preparation
- Annatto seeds, Red Piave variety (crop 2013) of the Station of the Grains Ltda. (São Paulo, Brazil) was used in the experiments. The defatting process was carried out in a pilot scale supercritical fluid extraction equipment (Thar Technologies, SFE-2×5LF-2FMC, Pittsburgh, USA). The oil removal with supercritical carbon dioxide as the solvent (99.9% CO2, Gama Gases Especiais Ltda., São Bernardo do Campo, Brazil) had the following conditions: T = 313 K, P = 200 bar, flow of CO2 = 200 g/min and solvent mass to feed mass ratio (S/F) = 11. The value of S/F was 68.6% lower than that used by Albuquerque and Meireles [24], and with it the seeds had the final condition of semi-defatted. The semi-defatted annatto seeds were stored in freezer (Metalfrio, model DA420, São Paulo, Brazil) at 263 K.
2.2. Chemical Characterization of Semi-Defatted Annatto Seeds and Active Solutions (AS)
- The determination of the chemical composition of the semi-defatted annatto seeds was performed at LAPEA/DEA/ UNICAMP (oil content) and at LASEFI/DEA/UNICAMP (total phenols and bixin contents).
2.2.1. Oil Content Determination
- The oil content of the semi-defatted annatto seeds was performed by the AOCS Official Method Ba 3-38 [25], before and after obtaining the AS. It was used an analytical balance (Sartorius analytic A200S, ± 0.0001 g, Sartorius GMBH Göttingen, Germany) for to weigh approximately 5 g of semi-defatted annatto seeds. Petroleum ether (Chemco, lot 29402, Sinergia, Campinas, Brazil) was used as an extracting solvent in the Soxhlet apparatus. A rotary evaporator (Heildoph Instruments, model 4001 Laborota, Viertrieb, Germany) vacuum was used for evaporation of the extracting solvent. The determination of the oil content was performed in two repetitions.The determination of the total phenols and bixin contents from the semi-defatted annatto seeds (before obtaining the AS) and of the AS were also performed as described below.
2.2.2. Total phenols Content Determination
- For the determination of total phenols content of the seeds and AS were determined by Folin-Ciocalteu method [26], with some adaptations to plant extracts suggested by Singleton et al. [27]. Total phenols was extracted for characterization of the raw material from the semi-defatted annatto seeds with hydroethanolic solvent (ethanol:water 10:90 v/v) by vortex agitation to enhance the efficiency of extraction during 5-8min. The obtained dry extracts by active solution solvent removal were diluted in hydroethanolic solvent (ethanol:water 10:90 v/v) to the concentrations appropriate for analysis. It was used 20 mg of dry extract (product from AS after evaporation of the extracting solvent) with an analytical balance (Sartorius Analytic A200S, ± 0.0001 g, Sartorius GMBH Göttingen, Germany). Gallic acid (Sigma-Aldrich, lot 023K 01171, USA), ethanol (Chemco, lot 26894, Sinergia, Campinas, Brazil) and distilled water were used to prepare the calibration solutions.The absorbance was read at UV-vis spectrophotometer (Hitachi, model U-3010, Tokyo, Japan) using measurement of 760 nm wavelength. For the purpose of this determination, the absorbance readings were done in three replicates.
2.2.3. Bixin Content Determination
- The bixin content of the seeds and AS were determined according to the set of monographs FAO/WHO experts Committee on food additives [28] and adapted by Albuquerque and Meireles [24]. Both the spectrophotometer and analytical balance were the same used in the determination of total phenolic content. The obtained dry extracts by active solution solvent removal were diluted in acetone to the concentrations appropriate for analysis. Thus, it was weighed approximately 1 mg of dry extracts (product from AS after evaporation of the extracting solvent). The absorbance was read in three replicates, at measure 487 nm wavelength. The bixin content of the seeds and AS was calculated in accordance with the law of Lambert-Beer, using the coefficient estimate [24]:
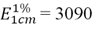
2.3. Process Description
- Two main pieces were coupled to shape the novel selective extraction method to produce the active solutions. A schematic diagram of the coupling of the equipments used to obtain the AS can be seen in Figure 1. The first (drawn in blue color) is from IKA using their Ultra-turrax® (Dijkstra Vereenigde, IKA® magic LAB®, NJ Lelystad, Holland) rotor-stator technology and the second (drawn in gray color) is an ultrasonic mixing system from Unique Group (Desruptor, Indaiatuba, Brazil). It should be consider that in the present work it is the first time the Ultra-turrax has been used for the extraction of AS from any plant material.
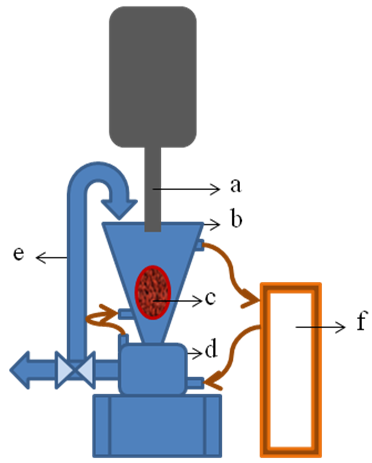 | Figure 1. Novel selective extraction method to produce AS |
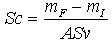 | (2) |
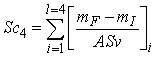 | (3.1) |
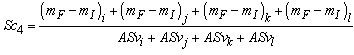 | (3.2) |
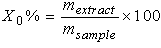 | (4) |
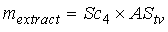 | (5) |
2.4. Experimental Design and Statistical Analysis
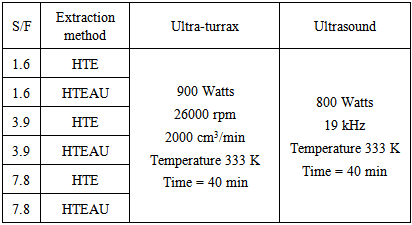
- To validate the novel extraction method to produce AS using semi-defatted annatto seeds as a model plant material and ethanol as extracting solvent, as well as was also prepared the Table 2, according to the following criteria, it was analyzed the technical limitation of the coupling of the units, both the Ultra-turrax and ultrasound. Thus, it was decided to consider the development of extractions with solvent mass to feed mass ratio (S/F) = ethanol mass/raw material mass at minimum, intermediate and maximum values. Taking into consideration that the ultrasound probe is immersed in solvent 3 cm and at the same time is free of contact with polyester bag that contains the semi-defatted annatto seeds.
|
3. Results and Discussion
3.1. Chemical Characterization of Semi-defatted Annatto Seeds and Active Solutions (AS)
- The determination of total phenols and bixin content in the active solutions to the quotient of solvent mass to feed mass ratio (S/F) 7.8, was rejected due to projected future uses of this solution. For example, for the extraction processes with organic solvents, which interest is to obtain a high concentration of solid inside the extracted product, a low or intermediate quotient of S/F is wished. In the opposite case, it will have to assume the additional costs to which it bears later another necessary process to concentrate the product extracted through evaporation processes.The content of oils of the semi-defatted annatto seeds before obtaining the AS was 1.24 % (wet basis, w.b.). The HTEAU, appears selective in the retention of oils in the seeds (Figure 2a), this selectivity is still more notorious when a ratio S/F is used to 1.6. So, the quantity of oils kept in the seeds was of approximately 40 %, whereas for S/F 7.8 of HTE the quantity of oils kept in the seeds was approximately 9%, the error bars represent the amplitude.
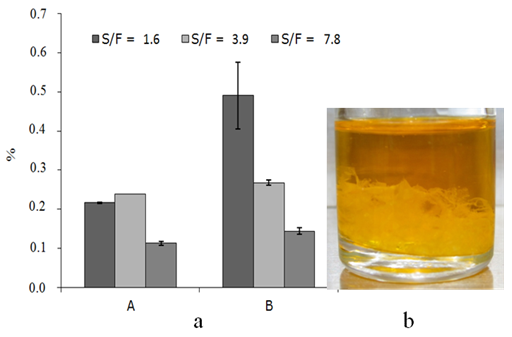 | Figure 2. a) Oil content in seeds (%, g/100 g seed oil on wet base) after extraction to obtain AS where A = HTE and B = HTEAU, b) Appearance of the oil after removal by the Soxhlet method |
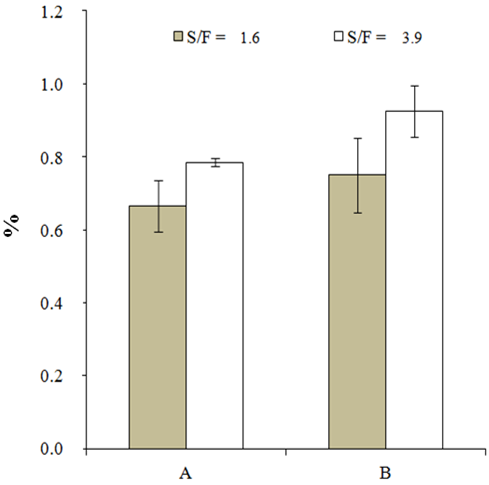 | Figure 3. Extraction efficiency of total phenols content from AS extracts, where A = HTE and B = HTEAU (%, mg GAE/100 mg of extract) |
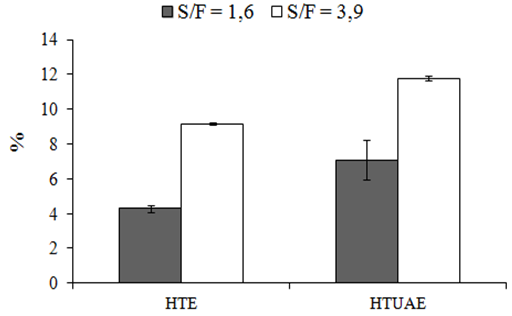 | Figure 4. Extraction efficiency of bixin from AS extracts, where A = HTE and B = HTEAU (%, mg bixin/100 mg of extract) |
3.2. Effects of the Extraction Method and Solvent Mass to Feed Mass Ratio (S/F)
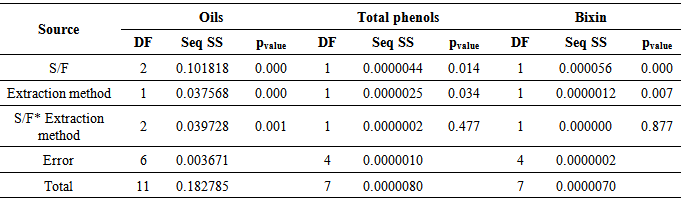
- The coupling of ultrasound probe of ultra-turrax equipment as a novel extraction method to produce AS, proved to be selective with regard to obtaining bixin and phenolic and oil retention in the seed semi-degreased. This selectivity is confirmed through the analysis of variance (ANOVA) observed in Table 3.
|
3.3. Literature Comparision
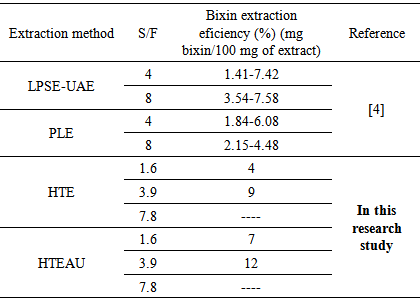
- Thus, Table 4 shows a comparison between bixin extraction eficiencies of those researches with the results obtained in this research. When compared the results of extraction efficiency of bixin of this research with literature information using the same extracting solvent and different extraction methods, it can be seen that our proposed process is very promising Therefore, it should be noticed that the content of oil in the extracts is also high and that extraction efficiency is a function of time.
|
4. Conclusions
- A hybrid technique coupling an Ultra-turrax® rotor-stator technology and an ultrasonic mixing system was successfully developed. Named by High Turbulence Extraction Assisted by Ultrasound (HTEAU) this novel process was described in detail using semi-defatted annatto seeds (Bixa orellana L.) as plant material and ethanol as extracting solvent as a model case. Additionally, the effects of extraction method and solvent mass to feed mass ratio (S/F) on oils, phenols and bixin recoveries were evaluated by analyses of variance (ANOVA). The biggest ratio S/F and the HTEAU method increased the content of total phenols and bixin in AS. Further experiments will be done using different raw materials in order to confirm the observed improvements by the coupling of both equipments.
ACKNOWLEDGEMENTS
- The authors are grateful to FAPESP (2012/10685-8) for financial support. R. Abel C. Torres thanks CNPq (190424/2012-5) for a MSc. assistantship and Rosa Koretzky for the translation of this article. Diego T. Santos thanks FAPESP (10/16485-5) and CAPES for the postdoctoral fellowships. M. A. A. Meireles thanks CNPq for a productivity grant (301301/2010-7).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML