-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2015; 5(1): 23-28
doi:10.5923/j.fph.20150501.04
Microbiological Assessment of Food and Hand-Swabs Samples of School Food Vendors in Benin City, Nigeria
Okareh O. T., Erhahon O. O.
Department of Environmental Health Sciences, Faculty of Public Health, College of Medicine, University of Ibadan, Ibadan, Nigeria
Correspondence to: Okareh O. T., Department of Environmental Health Sciences, Faculty of Public Health, College of Medicine, University of Ibadan, Ibadan, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
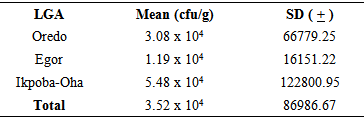
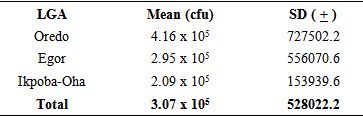
Sixty food vendors from public secondary schools drawn from the 3 Local Government Areas namely: Oredo (OD), Egor (EG) and Ikpoba-Oha (IK) in Benin City were selected for microbiological assessment of their food and hand-swabs samples. Aerobic Colony Count (ACC) <105 cfu/g was regarded as marginal limits of acceptable microbiological quality for food based on standard methods of International Commission on Microbiological Specifications for Foods. Most of the food samples (93.3%) had acceptable microbiological quality but indicated some level of contamination. Predominant microbial species of public health importance found in food samples were Staphylococcus aureus (30%) and Bacillus cereus (25%) while the mean ACC in food samples was OD: 3.08 x 104 cfu/g, EG:1.19 x 104cfu/g, IK: 5.48 x 104cfu/g. Microbial species found in hand-swab samples were Staphylococcus aureus (38.3%) and Staphylococcus epidermidis (21.7%) while the mean ACC in hand-swab samples was OD: 4.16 x 105cfu, EG: 2.95 x 105cfu, IK: 2.09 x 105cfu indicating a high microbial load.
Keywords: Food vendors, Microbiological quality, Microbial species, Hand- Swabs
Cite this paper: Okareh O. T., Erhahon O. O., Microbiological Assessment of Food and Hand-Swabs Samples of School Food Vendors in Benin City, Nigeria, Food and Public Health, Vol. 5 No. 1, 2015, pp. 23-28. doi: 10.5923/j.fph.20150501.04.
1. Introduction
- Food-borne diseases are diseases resulting from ingestion of bacteria, toxins and cells produced by microorganisms present in food (Doyle and Evans, 1999). Food-borne illnesses impose a substantial economic and quality of life burden on society by way of acute morbidity and chronic sequelae (Duff et al, 2003). Food-borne diseases encompass a wide spectrum of illnesses and are a growing public health problem worldwide. They are the result of ingesting contaminated foodstuffs, and range from diseases caused by a multitude of microorganisms to those caused by chemical hazards. The most common clinical presentation of food-borne diseases takes the form of gastro-intestinal symptoms, but such diseases can also lead to chronic, life-threatening symptoms including neurological, gynecological or immunological disorders as well as multi-organ failure, cancer and death.Food-borne disease is attributed to a wide variety of bacteria, parasites and viruses. It is found worldwide and cause human illness just about everywhere (Scott and Sockett, 1998; Tauxe, 1998; WHO, 1998). While the pathology, disease spectrum and causative agents differ, the same basic disease risk factors influence transmission. Although numerous control strategies are in place, person-to-person disease transmission has not ceased. Food handlers play an important role in ensuring food safety throughout the chain of production, processing, storage, and preparation (Hedberg et al, 1994; Goh, 1997; WHO, 1998). Approximately 10 to 20% of food-borne disease outbreaks are due to contamination by the food handler. The mishandling of food and the disregard of hygienic measures enable pathogens to come into contact with food and, in some cases, to survive and multiply in sufficient numbers to cause illness in consumers. Personal hygiene and environmental sanitation are key factors in the transmission of food-borne diseases. Investigations of outbreaks of food-borne disease throughout the world show that, in nearly all instances, they are caused by the failure to observe satisfactory standards in the preparation, processing, cooking, storing or retailing of food (Yew et al, 1993; Merican, 1997; Luby et al, 1998; WHO, 1988b).In most countries, the most common food–borne illness is Staphylococcus food intoxication (Talaro et al., 1996). The most commonly recognized food-borne infections are those caused by the bacteria Campylobacter, Salmonella, and E. coli O157:H7, and by a group of viruses (CDC, 2010). Staphylococcus spp has pathogenic strains which could cause food poisoning due to the heat stable Staphyloccal enterotoxin which is resistant to gastrointestinal enzymes. Campylobacter is a bacterial pathogen that causes fever, diarrhea, and abdominal cramps. It is the most commonly identified bacterial cause of diarrheal illness in the world. It is known to be gram negative rod bacteria and could be transmitted by oral route from food or drink, or contact with infected animals or animal products (Jawertz et al, 2004). Salmonella is also a bacterium that is widespread in the intestines of birds, reptiles and mammals. It can spread to humans via a variety of different foods of animal origin. The illness it causes, salmonellosis, typically includes fever, diarrhea and abdominal cramps. In persons with poor underlying health or weakened immune systems, it can invade the bloodstream and cause life-threatening infections (CDC, 2010). Eschericia coli is a member of the normal intestinal flora. Other normal bacterial flora of the intestine includes Klebsiella, Citrobacter, Serratia, Proteus, Enterobacter, e.t.c. They usually do not cause disease and in the intestine they may even contribute to the normal function and nutrition. However, E. coli could also cause clinically important infections. The bacteria only become pathogenic when it reaches tissues outside of their normal intestine or less common normal flora sites. E. coli found in water or milk is accepted as proof of fecal contamination from sewage or other sources (Jawertz et al, 2004). Shigella spp. causes bacillary dysentery. Shigella infections are almost always limited to the gastrointestinal tract and are highly communicable. Shigella produces a heat-labile exotoxin that affects both the GIT and the central nervous system.There is a dearth of information on the microbiological status of food, hand-swab and water samples in public schools in Benin City, Nigeria. This development makes it imperative to have reliable baseline data for improved school health status in Benin City. This study was therefore, undertaken to assess the bacterial burden of the food, hand-swabs and water samples of school food vendors in public secondary schools in Benin-City.
2. Materials and Methods
- Study AreaThis study was carried out in Benin City. Benin City is a densely populated city with 3 Local Government Areas (LGAs), namely: Oredo, Egor and Ikpoba-Oha. Benin City serves as the seat of government for Edo state. Major languages spoken are English, Pidgin English and Edo. There are 38 public secondary schools in Benin City. Exactly 25% of the food handlers from the public secondary schools in Benin-city were recruited for this study. A 3-stage sampling technique was adopted to select sixty (60) food vendors for this study. Sample CollectionThe food samples were collected with the dishing spoons used by the food vendors, packaged into sterile polythene bags and tied carefully. The hand-swab samples were collected by swabbing the palms of the food vendors with sterile swab sticks moistened in 0.1% peptone water. The water samples were collected from the storage containers used by the food vendors into sterile universal bottles. Sterile plastic containers were used for transporting the samples daily. These containers were swabbed with cotton wool soaked in ethanol, daily before usage.Microbial analysisThe microbiological analyses of food and hand-swab samples include aerobic colony count (ACC), isolation and identification of pathogens present. Aerobic Colony Count (ACC)Each food sample (10g) was weighed into a mortar and ground with a sterile pestle. Volume of distilled water (90ml) was poured into the mortar and the mixture was homogenized. Ten (10) ml of the mixture was then transferred to a test-tube and followed by serial dilutions. Serial dilutions of 10-1, 10-2 and 10-3 were made. Exactly 0.1ml of serial dilutions 10-2, 10-3 and 10-4 were cultured on Nutrient Agar petri and Saboroud Dextrose Agar (fungal plate count dishes using Miles and Misra method (Miles and Misra, 1938; Thacther and Clark, 1968; Thomas, B.T et al, 2012). The petri dishes were incubated at 37℃. The number of colonies seen were counted using a colony counter and recorded as colony forming unit per gram (cfu).Isolation and identification of Bacteria A wire loop, sterilized by flaming on a Bunsen burner, was used to inoculate the food samples onto Blood Agar, MacConkey Agar and Eosin Methylene Blue Agar using streak-plate method and then incubated for 37℃ for 24 hours. Media used were prepared according to the manufacturer’s instructions. After the incubation time, the different culture plates were examined for microbial growth. The morphology of the isolates was observed both macroscopically and microscopically, and then recorded. The isolates were made to undergo further biochemical tests for proper identification of the isolates. The biochemical tests carried out include Gram staining test, oxidase test, citrate test, catalase test, coagulase test, urease test and Indole test. Swab sample analysisThe swab-stick samples were first inoculated on Blood Agar, Macconkey agar, Eosin Methylene Blue agar and Saboroud Dextrose Agar (for fungal analysis) medium and these were incubated at 37℃ for 24 hours (for bacterial identification) and room temperature for 4 days (for fungal identification). After these appropriate incubations, the colonies were identified based on their morphological, physiological and biochemical features using microscope and standard biochemical methods.Aerobic colony count of swab samplesThe swab-sticks were placed individually in test-tubes containing 10ml of sterile distilled water and left standing for 30 minutes. This was then mixed thoroughly in 90ml of distilled water. Ten (10) ml of the mixture was then transferred to a test-tube and this was followed by serial dilutions. Serial dilutions of 10-1, 10-2, 10-3 and 10-4 were made. Exactly 0.1ml of serial dilutions 10-2 10-3 and 10-4 were cultured on Nutrient Agar and Saboroud Dextrose Agar (fungal plate count) petri dishes, using drop plate method or Miles and Misra method (Miles and Misra, 1938; Thacther and Clark, 1968; Thomas, B.T et al, 2012). The petri dishes were then incubated at 37℃ for 24 hours (bacterial aerobic colony count) and room temperature for 4 days (fungal aerobic colony count). The number of colonies seen were counted using a colony counter and recorded as colony forming unit per gram (cfu).Data analysisThe number of colony forming unit per gram (cfu/g), per milliliter (cfu/ml) was calculated by standard methods. All data generated from this study was entered into the computer using SPSS 15 software.(Statistical Package for Social Sciences). Analysis was done at two levels. The various means, median and modes were calculated and displayed using frequency tables, bar charts and histograms. The second level of analysis was cross-tabulations. Chi-square test was used to investigate the statistical significance of the associations between two qualitative variables. This was done at 5% level of significance.
3. Results and Discussion
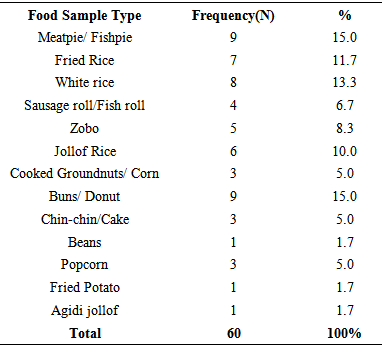
- The results of the food and hand-swab samples collected from the sixty food handlers in the 3 Local Government Areas in Benin-city that were recruited for the laboratory analysis is as shown in Table 1 and Fig.1. Some of the food samples collected and analyzed were meat pie/ fish pie (15%), buns/donut (15%), white rice (13.3%) and fried rice (11.7%).
|
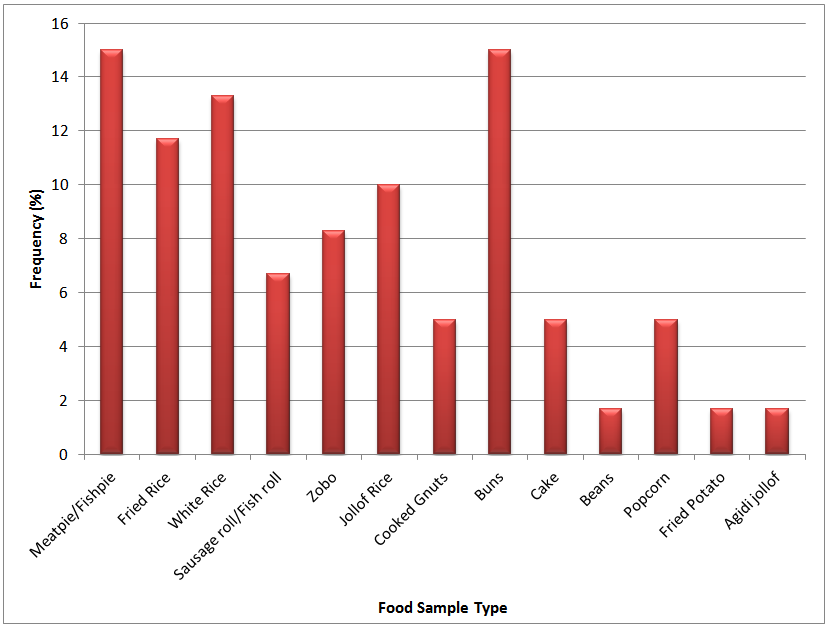 | Figure 1. Food Sample Types and Frequency of Sampling |
|
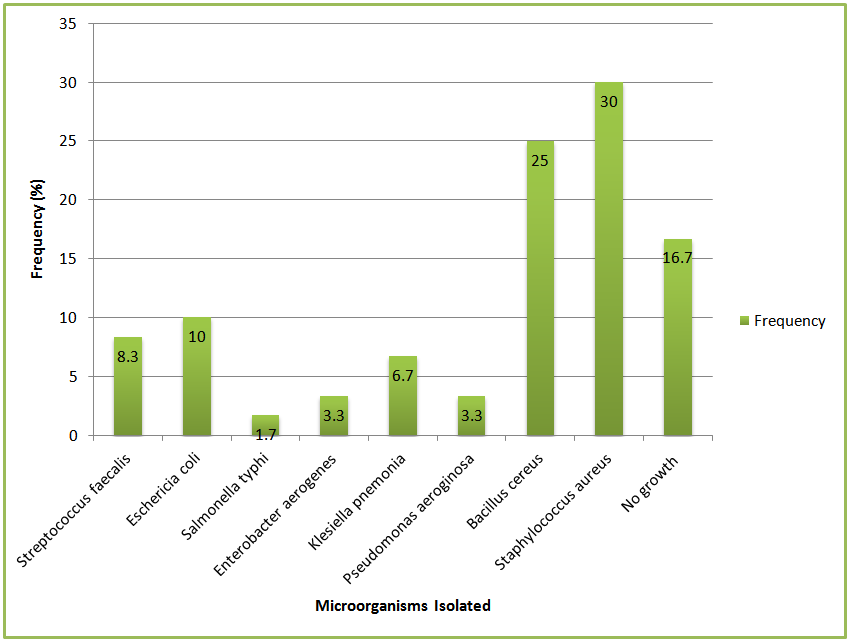 | Figure 2. Microorganisms isolated from the food samples |
|
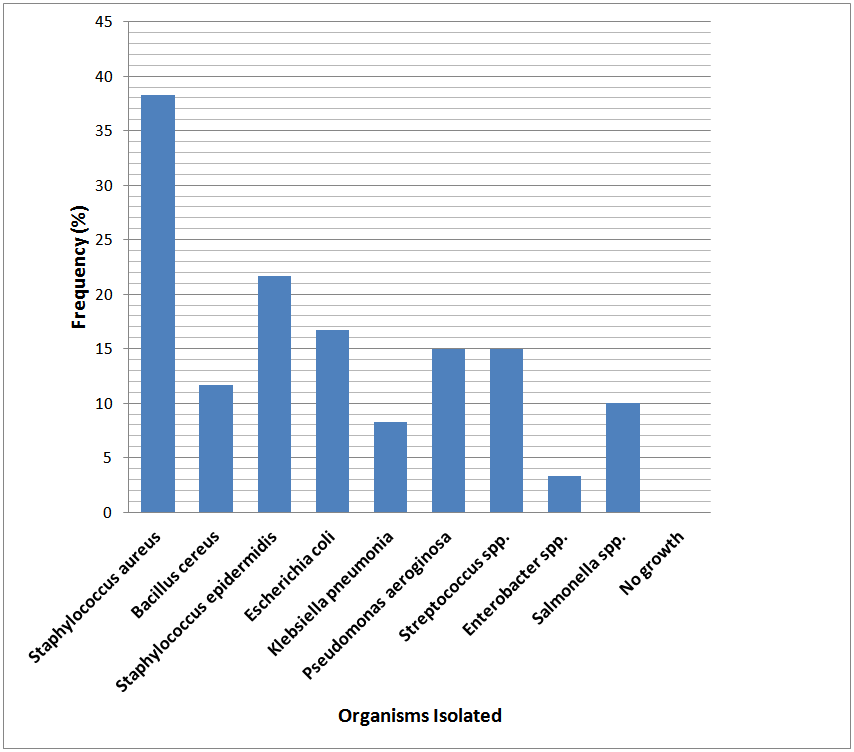 | Figure 3. Microorganism isolated from the hand-swab samples |
4. Conclusions
- The microorganisms in the vendors’ food were within acceptable microbiological limits or borderline limits. However, the microorganisms isolated from hand-swabs of the food vendors show possible hygiene problems in food preparation or handling of the food or utensils. Microorganisms such as Staphylococcus aureus, Escherichia coli, Bacillus cereus e.tc found in the food and hand-swab samples could lead to acute or chronic food poisoning or food-borne illnesses such as gastroenteritis etc. These organisms are entirely preventable by practicing good sanitation and proper food handling techniques. To prevent an incidence of food contamination and intoxication in foods, there is need to educate and advocate for the importance of environmental sanitation and good food handling practices especially proper hand-washing practices among school food vendors.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML