-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2015; 5(1): 17-22
doi:10.5923/j.fph.20150501.03
Effect of Diet Supplementation with Some Food Industry by- products on Cholesterol Levels of Rats
Abdel Moneim E. Sulieman 1, Wisal A. M. Babiker 2, Sirekhatim B. Elhardallou 3, Elamin A. Elkhalifa 4
1Department of Biology, Faculty of Science, University of Hail, Kingdom of Saudi Arabia
2Faculty of Sciences, University of Taif, Taif, Kingdom of Saudi Arabia
3Faculty of Applied Medical Sciences, University of Taif, Taif, Kingdom of Saudi Arabia
4Department of Food Science and Technology, Faculty of Engineering and Technology, University of Gezira, Sudan
Correspondence to: Abdel Moneim E. Sulieman , Department of Biology, Faculty of Science, University of Hail, Kingdom of Saudi Arabia.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
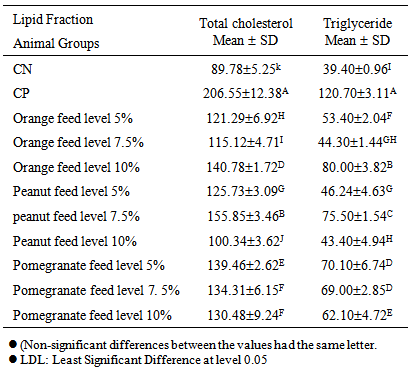
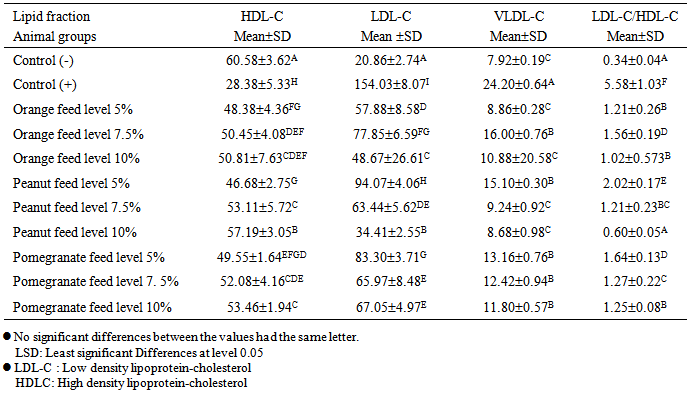
Effect of food industry by-products orange peels, peanut skin peels and pomegranate peels on cholesterol level was investigated using sixty male adult Sprague-Dawely rats weighing 125 ± 5g. The negative control group of rats was fed on basal diet while the other sub-groups (positive groups) were fed on basal diet and the cholesterol diet (which was prepared by replacing 5% corn oil by cholesterol) together with various formulations of food by-products; orange peels, peanut skin and pomegranate peel. Total cholesterol and triglycerides decreased significantly (P<0.05) when rats fed on basal diet containing high doses of the food by-products. The total cholesterol and triglycerides levels pronouncedly decreased in rats fed on 10% peanut skin (100.34 ± 3.62 and 43.4 ± 4.94), respectively followed by 5% peanut skin (125.73 ± 3.09 and 46.24 ± 4.63). The serum HDL-C decreased significantly (P<0.05) in (control +) that fed on hypercholesterolemic diet (28.38 + 5.33), compared with healthy rats (control - ) that fed on basal diet (60.58 ± 3.62). The highest mean values of the ratio between LDL -C/HDL-C for tested groups were observed in the group fed on hypercholesterolemic diet containing 5%, followed by 7.5%, l0% pomegranate peel and 5% peanut skin. While, the lowest mean values of was observed in the group fed on basal diet containing the orange feed level 7.5%.
Keywords: Cholesterol, Lipids, Peanuts, Dietary fibre
Cite this paper: Abdel Moneim E. Sulieman , Wisal A. M. Babiker , Sirekhatim B. Elhardallou , Elamin A. Elkhalifa , Effect of Diet Supplementation with Some Food Industry by- products on Cholesterol Levels of Rats, Food and Public Health, Vol. 5 No. 1, 2015, pp. 17-22. doi: 10.5923/j.fph.20150501.03.
Article Outline
1. Introduction
- Cholesterol is a fat-related compound; its structure is different from the triglycerides and does not exist in plant foods. Cholesterol is essential for the synthesis of bile, sex hormones, cortisone and vitamin D and is needed by every cell in the body. The body manufactures 800-1000 mg of cholesterol a day in the liver. The advisable blood cholesterol levels do not exceed 200 mg/dl (200 milligrams of cholesterol per one deciliter of blood). It is recommended that daily cholesterol intake not exceed 300 mg [1].The plasma total cholesterol concentration ranges from 150 to 250 (average 200) mg/dl. Cholesterol is transported in the blood plasma in the form of lipoprotein; the greater proportion (100-180 mg/dl) is associated with the low-density lipoprotein (LDL). Smaller amounts are associated with high-density lipoprotein (HDL) (40-70 mg/dl) and least is associated with very low-density lipoprotein (VLDL) (10-30 mg/dl) [2]. Fruits and vegetables have been known to contain a variety of antioxidant components. It has been suggested that antioxidants may protect biomolecules from oxidative damage and therefore be associated with reduced risks of cardiovascular disease and certain cancer. It has been reported that there has been a inverse relationship between fibre intake and cardiovascular diseases [3]. A protective association was found between fruit and vegetable consumption and the risk of chronic heart disease [4] with relative risks ranging from 0.83 - 0.93 for the highest to the lowest fruit and vegetable intakes. A recent pooled case-control study, including data from 52 countries, found a 30% lower risk of m yocardial infarction in those with the highest intakes of fruits or vegetables [5]. Dietary supplementation with nutrients rich in antioxidants is associated with inhibition of atherogenic modifications to LDL, macrophage foam and atherosclerosis. There are some nutritional wastes such as pomegranate peel, orange peel and peanuts skin (Arachis hy pogaea L) are considered important factors of therapeutic diets or as diabetic diet and nutrient effect supping essential nutrient elements such as fiber, vitamin, and mineral to human body. These by-products are rich in nutrients such as vitamins and minerals beside high contents of dietary fibres. These by-products have been demonstrated to have high antioxidant activity and effective in prevention of atherosclerosis, especially pomegranate granatum) extracts which have been shown to possess significant antioxidant activity in various in vitro models This study aims to investigate the effect of the food industry by-products of orange peels, peanut shell, and pomegranate peel on the hypercholesterolemic rats.
2. Materials and Methods
2.1. Samples
- The materials used for the current study were three food industry by-products namely orange (Citrus sinenis) peels, peanut (Arachis hypogaea L) skin peels and pomegranate (Punica granatum) peels. These by-products peels were brought form the local market, well washed and dried at 63℃ using a fan oven. They were then processed into fine powder.
2.2. Experimental Animals
- Sixty male adult Sprague-Dawely rats weighing 125± 5g which were purchased from the Research Institute of Ophthalmology, Giza, Egypt were used for the biological evaluation. Each rat was housed in a special cage under controlled conditions. The animals were observed daily for the apparent signs such as shape, color and distribution of hair and physical activity. All rats were fed for 3 days on the standard diet for adaptation before the beginning of the experiment. All rats were provided with water by glass tubes through wire cage and fed as needed throughout the experimental period.
2.3. Preparation of Hypercholesterolemic Rats
- Sixty rats were fed on the cholesterol diet which was prepared by adding cholesterol at 5% level by replacement within corn oil. Cholesterol was dissolved in hot corn oil before being thoroughly mixed into the diet [6].
2.4. Experimental Diet
- The composition of basal diet and experimental diets per 100g contained 12g casein, 10g corn oil, 4g salt mix, 1g vitamin mix, 0.2g Calcium chloride and 7.8g corn starch. The experimental diets contained in addition 5%, 7.5% and 10% orange peels, peanut peels and pomegranate peels.
2.5. Grouping of Experimental Animals
- Rats were divided into 11 groups, 6 rats in each group and fed with experimental diets for consecutive 28 days as follows:Group 1 Normal rats, were fed on basal diet as control negative (-).Group 2 Hypercholesterolemic rats, were fed on basal diet as control positive (+).Group 3 Hypercholesterolemic rats, were fed on basal diet supplemented with 5% Orange peel powder.Group 4 Hypercholesterolemic rats, were fed on basal diet supplemented with 5% peanut peel powder. Group 5 Hypercholesterolemic rats, were fed on basal diet supplemented with 5% Pomegranate peel powder.Group 6 Hypercholesterolemic rats, were fed on basal diet supplemented with 7% Orange peel powder. Group 7 Hypercholesterolemic rats, were fed on basal diet supplemented with 7% peanut Peel powder.Group 8 Hypercholesterolemic rats, were fed on basal diet supplemented with 7% Pomegranate peel powder.Group 9 Hypercholesterolemic rats, were fed on basal diet supplemented with 10% Orange peel powder. Group 10 Hypercholesterolemic rats, were fed on basal diet supplemented with 10% peanut peel powder. Group 11 Hypercholesterolemic rats, were fed on basal diet supplemented with 10% Pomegranate peel powder.
2.6. Blood Sampling
- At the end of the experiment, rats were fasted for overnight (more than 12 hours) and anesthetized with chloroform. Blood samples were collected in clean dry centrifuge tubes from hepatic portal vein and centrifuged for 10 minutes at 3000 rpm to separate the serum, which was kept in tubes at -18°C till analysis.
2.7. Blood Lipids Tests
- a- Determination of total lipidsThe total lipids in serum were calorimetrically determined according to Zollner and Kirsch [7] method in which lipids react with sulfuric, phosphoric acids and vaniline to form pink colored complex. The absorbance of sample (A sample) and standard (Astandard) were read against reagent blank within 30 min, at 454nm. The total lipids concentration was calculated according to the following formula.
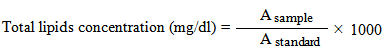 b- Determination of CholesterolEnzymatic colorimetric method was used for the determination of cholesterol in serum according to Richmond [8]; and Allain [9]. c- Determination of high density lipoprotein Cholesterol (HDL-C) Enzymatic colorimetric method was used to determine HDL- cholesterol according to Albers et. al., (1983) [10].d- Determination of very low density lipoprotein (VLDL-C) The LDL-C was determined according to Friedwald et. al. [11]. The VLDL-C was calculated as follows:VLDL-C = trigelycerides/5e- Determination of low density lipoprotein cholesterol (LDL-C)The VLDL-C was determined according to Friedwald et. al. (1972). The LDL-C was calculated as follows:LDL-C = HLD-C-VLDL-C
b- Determination of CholesterolEnzymatic colorimetric method was used for the determination of cholesterol in serum according to Richmond [8]; and Allain [9]. c- Determination of high density lipoprotein Cholesterol (HDL-C) Enzymatic colorimetric method was used to determine HDL- cholesterol according to Albers et. al., (1983) [10].d- Determination of very low density lipoprotein (VLDL-C) The LDL-C was determined according to Friedwald et. al. [11]. The VLDL-C was calculated as follows:VLDL-C = trigelycerides/5e- Determination of low density lipoprotein cholesterol (LDL-C)The VLDL-C was determined according to Friedwald et. al. (1972). The LDL-C was calculated as follows:LDL-C = HLD-C-VLDL-C2.8. Statistical Analysis of Data
- Data were statistically analyzed using computerized program Scientific Computer Center, Faculty of Home Economics, Minufyia University, using Duncan Multiple Range Test (one way ANOVA test) according to Amitage and Berry [12].
3. Results and Discussion
3.1. Lipid Profile of Hypercholesterolcmic Rats
- Table (1) illustrates the effect of feeding different levels from orange peels, peanut skin and pomegranate peel on the serum cholesterol and triglycerides levels of hypercholesterolemic rats. Data in this table showed that total cholesterol and triglycerides levels (mg/dl) increased significantly (P<0.05) for rats fed on hypercholesterolemjc diet (control positive), compared to healthy rats fed on basal diet (control negative) (206.55 ± 12.38 and 120.7 ± 3.11) vs. (89.78 ± 5.28 and 39.40 ± 0.96).
|
3.2. Effect of Feeding on Cholesterol Fractions of Hypercholesterolemic Rats
- Results in Table (2) exhibit the effects of three levels (5%, 7.5% and 10%) from nutritional wastes on cholesterol fractions i.e, high density lipoprotein (HDL-C), low density lipoprotein (LDL-C), very low density lipoprotein (VLDL-C) and the ratio between LDL-C/HDL-C of hypercholesterolemic rats.
|
3.3. High Density Lipoprotein-Cholesterol (HDLC)
- Results in Table (2) showed that, the serum HDL-C decreased significantly (P<0.05) in (control +) that fed on hypercholesterolemic diet (28.38 + 5.33), compared with healthy rats (control -) that fed on basal diet (60.58 ± 3.62). On the other hand the mean values of serum HDL-C of all treated rats with orange peels, peanut skin and pomegranate peel increased and decreased significantly (P<0.05), as compared to control (+) and control (-), respectively.Concerning levels of nutritional food industry by-products, the highest mean value of HDL-C (57.19± 3.05) was obtained when hypercholesterolemic rats were fed on diet containing 10% peanut skin, while the lowest mean values of the same parameter (46.86 ±4.36) was obtained when hypercholesterolemic rats were fed, diet containing 5% orange peels. On the other hand, feeding hypercholesterolemic rats on diet containing, orange peel level 5% peanut skin showed significant decrease (P
3.4. Low Density Lipoprotein-Cholesterol (LDL-C)
- The mean values of low-density lipoprotein cholesterol of normal and hypercholesterolemic rats that fed on nutritional peels are summarized in Table (2). The results indicated that, feeding rats with hypercholesterolemic diet led to significant increase (P<0.05) in LDL-C, compared with control negative group that were fed on basal diet.Low density lipoprotein-cholesterol (LDL-C) of all treated rats with basal diet containing nutritional wastes decreased significantly (P<0.05), compared with control+ Meanwhile, these treatments for rats led to increase LDL-C significantly, compared to (control - ).Diet induced an increase in LDL-C in serum rats, compared to normal hypercholesterolemic of rats. However, hypercholesterolemic rats that fed on 5, 7.5 or 10% orange peels showed a significant decreased in serum LDL-C levels, the maximum reduction showed in rats fed on basal diet containing 10% peanut skin, when compared to control (+) (34.41 ± 2.25 vs 154.03 ± 8.07). These results appear to be in agreement with those of Karvonen et al. [19] who found that LDIL-C decreased significantly due to feeding on orange peels. The same trend was found using nutritional peels. The data showed that the highest mean value of the same parameter was found in rats fed on basal diet containing peanut feed level 7.5%, pomegranate feed level. 5% and orange feed level 10%.From the above mentioned data, it could be concluded that, all treated rats with 5%, 7.5% and 10% nutritional peels resulted in significant decrease (P<0.05) than control (+) group. The best results in these treatments were observed in all groups fed on hypercholesterolemic diet containing orange peels, especially the hypercholesterolemic group of rats fed on diet containing oil extracted from orange feed level 7.5% [18].
3.5. Very Low-Density Lipoprotein-Cholesterol (VLDLC)
- Table (2) show the very low-density lipoprotein cholesterol in serum of hypercholesterolemic rats fed on diet containing 5, 7.5% and 10% orange peels, peanut skin and pomegranate peel compared with the control (+). The results in this Table showed that, the mean values of VLDL-C of rats that fed on basal diet containing cholesterol (hypercholesterolemic diet) increased significantly (P
3.6. The Ratio between LDL-C/HDL-C
- Table (2) also showed that serum LDL-C/HDL-c increased significantly P<0.05 in hypercholesterolemic (control +) that fed on diet (5.58 ± 1.03), compared with healthy rats control (-) that fed on basal diet only (034 ± 0.04). The mean values of the ratio between LDL-C/I-IDL-C of all treated rats with two levels (5%, 7.5% and 10% nutritional peels decreased significantly P<0.05, as compared to control (+).Concerning orange peels diets containing orange peels from 7.5% fed to hypercholesterolemic rats, led to significant increase of the ratio between LDL-C/HDLC, compared with the hypercholesterolemic groups fed on diet containing orange feed level 10. % and the orange feed level 5%. On the other hand hypercholesterolemic groups of rats fed on diet containing the amount of orange peels level 7.5% showed significant increase (P<0.05) in the ratio between LDL-C/VLDL-C, compared to control (+), while non-significant changes was observed in this ratio when compared with control (-).Using diets containing 5,7.5 and 10% peanut skin and pomegranate peel for hypercholesterolemic led to significant decrease and increase (P<0.05) in the ratio between LDL-C/HDL-C, compared with control (+) and control (-), respectively. This finding was similar to that recorded by Gyllling and Miettinen [20]. Who showed that, volatile oils dissolved in orange peels significantly reduced serum LDL-C by 15% and increase the HDL/LDL ratio by 27%.The highest mean values of the ratio between LDL -C/HDL-C for tested groups were observed in the group fed on hypercholesterolemic diet containing 5% pomegranate peel, followed by pomegranate peels level 7.5%, l0% pomegranate peel and 5% peanut skin. While, the lowest mean values of LDL C/HDL-C was observed in the group fed on basal diet containing the orange feed level 7.5%.From the above mentioned data it could be concluded that, total cholesterol, triglycerides, LDL-C, VLDL-C and the ratio between LDL/HDL-C were more decreased in hypercholesterolemic rats which were fed on orange peels. Some of the difference may be attributed to difference in the squalene and phytosterol contents of the oils.Table (2) exhibited the correlation between treatments, triglycerides and cholesterol fractions. The statistical analysis showed that there was non-significant. Correlation between treatments all parameters under study. Meanwhile correlation is highly positive significant (P<0.1) with, VLDL-C, LDL-C while highly negative correlation was found due to HDL-C. For the ratio between LDL/HDL-C. A similar trend was found. Concerning VLDL-C, LDL-C, HDL-C and the ratio between LDL/HDL which showed a positive correlation between all parameters except that of HDL which showed a negative correlation.Concerning HDL-C, as expected showed a highly negative correlation with parameters. From the above mentioned data, it could be concluded that some changes in the animal metabolism of the lipid may be found and this point must be taken into consideration in the further studies.
4. Conclusions
- This study was undertaken to investigate the effect of some food industry by-products on hyper-cholesterolemic rats. Blood lipid profile and cholesterol levels were reduced in case of pomegranate peel, followed by peanut red skin and orange peels when compared to control positive. The hypocholesterolemia properties of these by-products could be attributed to one or more of its photochemical including antioxidants. The study supports other research indicating that fruits by-products orange peels, peanut skin peels and pomegranate peels may have cholesterol-lowering properties and, therefore, possibly a role in the prevention of heart disease. It is highly recommended to incorporate dietary fiber such as pomegranate peel, peanut red hull and orange peels in the diets of hypercholestermic patients. Further research is needed to provide more evidence that will make it possible to understand the mechanisms behind these effects.
ACKNOWLEDGEMENTS
- The authors express their thanks to the member of the Department of Food Science and Technology of Gezira University, Sudan and the Research Institute of Ophthalmology, Giza, Egypt for their assistance presented by them.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML