-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2015; 5(1): 10-16
doi:10.5923/j.fph.20150501.02
Lipid Profile of Eggs from Laying Chickens Fed Five Proprietary Vitamin-Mineral Premixes under Two Rearing Systems as Influenced by Duration of Storage
Olugbenga Adeniran Ogunwole , Akinola Yinka Paul Ojelade , Emem Aquawo Essien , Mutiu Oyeyemi Oyewo
Agricultural Biochemistry & Nutrition Unit, Department of Animal Science, University of Ibadan, Ibadan, Nigeria
Correspondence to: Olugbenga Adeniran Ogunwole , Agricultural Biochemistry & Nutrition Unit, Department of Animal Science, University of Ibadan, Ibadan, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Effect of five different proprietary vitamin-mineral premixes (VMP) on lipid profile of eggs from laying chickens raised under two rearing systems (RS) in the duration of storage (DOS) was undertaken. Bovan nera pullets (n = 480) at week 18 (point of lay) were divided into two equal portions of 240 birds each for conventional battery cage (BC) and an open-sided deep litter (DL) housing system respectively. Birds were randomly allotted to five dietary treatments of 48 pullets per treatment both in the BC and DL. Each treatment was replicated 6 times with 8 pullets per replicate. Five isocaloric and isonitrogenous diets were formulated, each was supplemented appropriately with 0.25% of different VMP 1, 2, 3, 4 and 5 to obtain treatments T1, T2, T3, T4 and T5 respectively. The experimental diets and water were offered to birds ad libitum. The experiment was a 2 x 5 x 5 factorial arrangement in a completely randomised design. Effect of RS on yolk total cholesterol, high density lipoprotein, and very low density lipoprotein was similar (p<0.05). However, values of triglyceride and low density lipoprotein were significantly different (p<0.05). All yolk lipid indices varied significantly (p<0.05) with the type of dietary VMP as well as the interaction between RS and VMP type. The lipid peroxidation of eggs was higher (p<0.05) in the DL (0.034) compared with the BC (0.028). Egg thiobarbituric acid reactive substances (TBARS) (mg/dL) from hens fed T1, T2, T3, T4 and T5 were 0.028, 0.031, 0.033, 0.032 and 0.027 respectively and they varied significantly (p<0.05). Yolk TBARS for days 0, 7, 14, 21 and 28 were 0.021, 0.026, 0.033, 0.035 and 0.036 respectively also increased significantly (p<0.05) with DOS. TBARS regression on DOS gave positive and highly significant (p<0.01) linear equations: y = 0.00056x + 0.022; R2 = 0.842 for eggs from DL while y = 0.00056x + 0.019; R2 = 0.688 for those from BC. Therefore, individual effect and the interactions of VMP type, RS and DOS altered the lipid profile of eggs.
Keywords: Egg yolk, Total cholesterol, Thiobarbituric acid reactive substances, Deep litter, Battery cage
Cite this paper: Olugbenga Adeniran Ogunwole , Akinola Yinka Paul Ojelade , Emem Aquawo Essien , Mutiu Oyeyemi Oyewo , Lipid Profile of Eggs from Laying Chickens Fed Five Proprietary Vitamin-Mineral Premixes under Two Rearing Systems as Influenced by Duration of Storage, Food and Public Health, Vol. 5 No. 1, 2015, pp. 10-16. doi: 10.5923/j.fph.20150501.02.
Article Outline
1. Introduction
- The hen’s egg possesses an excellent nutritive value and constitutes a traditional food used in many basic and formulated preparations. Egg being a source of high-quality protein is used as a reference standard for evaluating the protein quality in all other foods as eggs scored 100% on the biological value chart [23]. Despite the nutritional value of egg, there are marketing challenges and health issues arising from its’ production, consumption, quality and storage over time. The quality and shelf-life of egg is of vital consideration to prevent deterioration by microorganisms which cause physical and chemical changes observed in egg after lay thereby making egg unfit for consumption especially in case of egg sales glut. The erstwhile phobia associated with the consumption of egg relates to its’ believed high cholesterol levels [8]. Lipids are sources of energy, components of cell membranes and serves as insulation for nerves constituting about 65% of the egg yolk with triglyceride having the highest (about 65%) component, 29% phospholipids, 5% cholesterol and less than 1% free fatty acids on dry matter bases [6]. However, eggs have been reported to be successfully enriched with desired compounds through dietary interventions [12] which reduced cholesterol levels. For instance, selenium supplementation in layers’ diet increased egg levels of vitamin E while increased dietary vitamins E and C alongside supplemental omega n-3 found in fish oils for poultry helped prevent lipid peroxidation of egg [5]. In Nigeria, the southwest which remained the hub of egg production habitually witnesses sporadic sales glut in one time or the other annually. Produced eggs are often not processed into powder or to the forms that can be stored for a long time. They must therefore be consumed within the shortest spate of their production. Fresh egg preservation and storage here then becomes so imperative as the only avenue of preventing heavy losses to farmers. Egg quality and durability have been directly linked to the nutrition of laying hen [17]. Among the dietary factors affecting the nutritional value of egg and keeping quality includes vitamins, minerals and fat. Vitamin-mineral premix (VMP) has long been identified as a critical input in the feeding of commercial layers as the use of quality premix is one of the main features of a successful poultry production leading to improved food safety, reliability and performance [19, 2] On the other hand, the management system of layers has also been observed to contribute to the chickens performance, egg quality and shelf-life [18]. Eggs from conventional battery cages (BC) were healthier and possessed longer storage capacity over those kept in deep litter (DL) or free range systems [3]. This attribute was linked to several advantages of BC such as separation of eggs from droppings, reduction of cracked eggs and lowered risks of diseased and infected eggs while better yolk and albumen quality was observed in DL system over eggs from layers kept in BC [16]. Rearing systems (RS) have also been reported to affect egg cholesterol content significantly [3]. Few reports on VMP use in commercial poultry production [13, 14, 15, 25] as well as effects of RS in poultry production [4, 16] have hitherto refused to link the effects of the different proprietary VMP condiments and the RS to variability in the lipid composition and storability of eggs in the hot humid tropics. Therefore, this study was aimed at evaluating the lipid profile of eggs from laying chickens fed five different proprietary VMP under two RS as influenced by duration of storage.
2. Materials and Methods
2.1. Experimental Location and Animals Allotment
- The experiment was carried out at the Teaching and Research Farm, University of Ibadan, Ibadan, Nigeria. A total of 480 Bovan nera chickens were randomly allotted to six dietary treatments of 48 chicks per treatment. Each treatment was a triplicate of eight birds per replicate. Birds (n=240) were each raised on conventional BC and a partitioned DL house respectively.
2.2. Experimental Diets
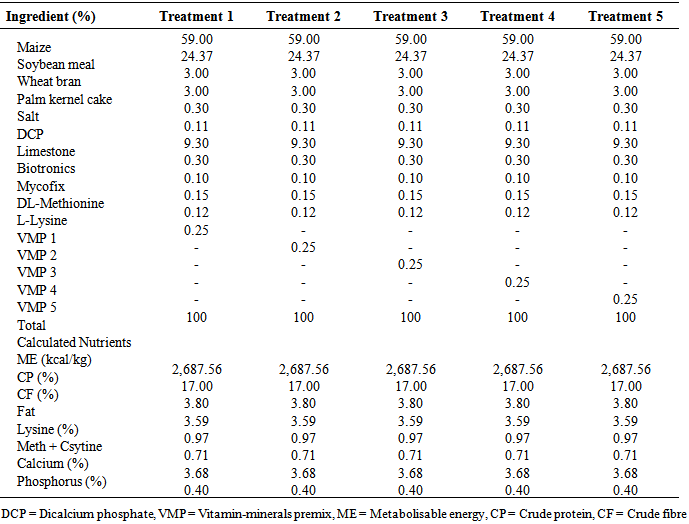
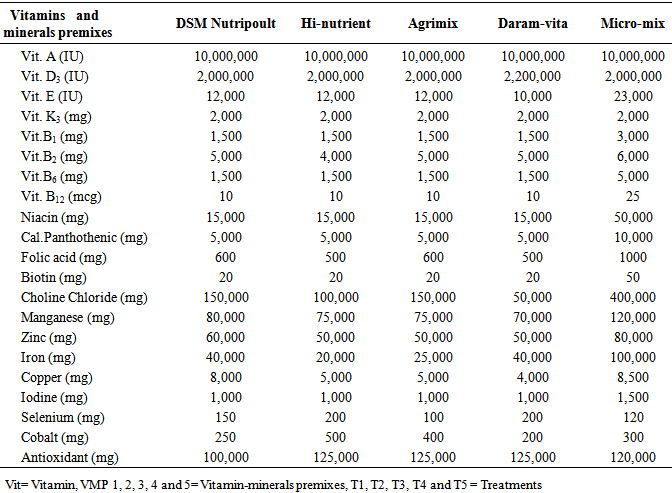
- Six isocaloric and isonitrogenous diets were formulated at both the starter and finishers’ phases. The five proprietary VMP were purchased from a commercial feed milling factory in Ibadan, Nigeria. Each diet was supplemented with 0.25% VMP as shown below:T1 - Basal diet + 0.25% DSM Nutripoult premix (VMP 1)T2 - Basal diet + 0.25% Hi-nutrient premix (VMP 2)T3 - Basal diet + 0.25% Agrimix premix (VMP 3)T4 - Basal diet + 0.25% Daramvita premix (VMP 4)T5 - Basal diet + 0.25% Micro-mix premix (VMP 5)The composition of the experimental layers’ diet is shown in Table 1 while Table 2 shows the composition of the test VMP.
|
|
2.3. Birds Management and Eggs Sampling
- Birds were weighed prior to their allotment to various dietary treatments. Routine medication, vaccination and husbandry practices were administered on them. The feeding period lasted 12 weeks within which the birds were given feed and water ad libitum. The design of the experiment was a completely randomized design in a 2 x 5 x 5 factorial arrangement. At week 30, three freshly laid eggs were randomly collected from each treatment in both RS.
2.4. Cholesterol and TBARS Determination
- Using a syringe, 3 - 5mls of the mixed egg was sampled into bottles with anticoagulant, ethylene diamine tetra-acetic acid for lipid profile analysis. The samples were centrifuged at 1800r/m and analysed using Hitachi 902: Auto Analyzer for total cholesterol, triglycerides, HDL, LDL and VLDL which was calculated by division of triacylglycerol by 5 in the respective egg yolk samples. Another set of 75 freshly laid eggs were immediately collected across the treatment in both RS and labeled appropriately for subsequent TBARS analyses. The remaining sixty eggs were stored at room temperature of 26℃ to observe egg keeping quality during storage periods of 7, 14, 21 and 28 days. The TBARS in the egg yolk was measured as the composition of malondialdehyde (MDA) in the egg sample using the formula:TBARS (mg MA/100g) = K x A, where: K = -9.242 and A = Absorbance.
2.5. Statistical Analysis
- Data were subjected to a two-way analysis of variance (ANOVA) at α 0.05 and α 0.01 using the General Linear Model procedure [22] while significant means were separated by Duncan multiple range option of the same software. Data were also subjected to regression analysis to establish relationships among the test variables.
3. Results and Discussion
3.1. Effect of RS on Egg Lipids
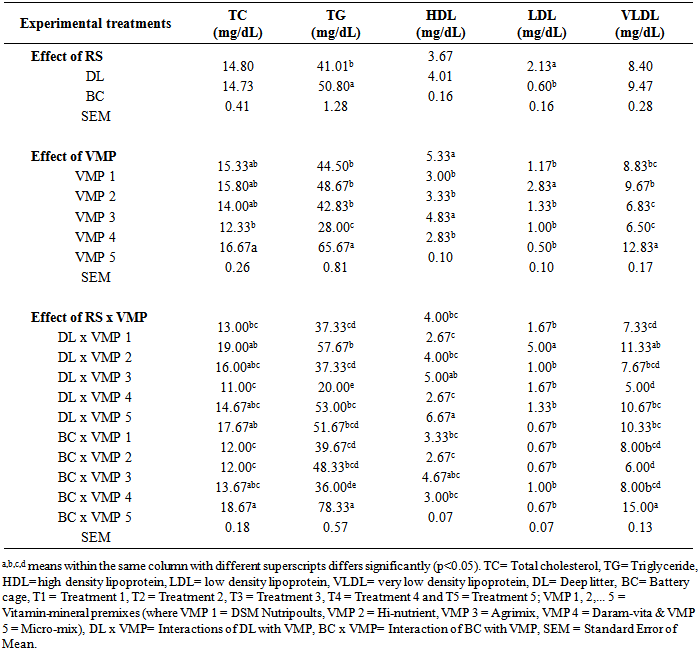
- Effect of five proprietary VMP on the lipid profile of egg yolk from hens in two RS is shown in Table 3. The RS, be it DL or BC did not have any significant effect (P>0.05) on the total cholesterol content of egg yolk. Similar values (P>0.05) of 14.80 and 14.73mg/dL were respectively obtained for the DL and BC eggs yolk. This could be because the chickens were able to maintain their egg cholesterol level which was a reported consideration for egg composition and embryo development [25]. This assertion as was earlier reported [11, 10] revealed no effect of RS (BC and DL) on egg yolk total cholesterol. Also, yolk high density lipoprotein (HDL, mg/dL) of 3.67 for DL and 4.01 for BC and very low density lipoprotein (VLDL) of 8.40 for DL and 9.47 for BC were not significantly different (p>0.05). However, triglycerides mg/dL (50.80 for BC and 41.01 for DL) and low density lipoprotein (LDL) mg/dL (2.13 for DL and 0.6 for BC) differed significantly (P<0.05). Higher egg yolk total cholesterol of hens in BC had been attributed to relatively reduced activity of birds which leads to increased fat deposition [20]. However, there was report [11] of less fat in the yolk of hens from free range compared to indoor housing.
|
3.2. Effect of Dietary VMP on Egg Lipids
- Effect of the different dietary proprietary VMP on all lipid indices were significant (P<0.05). Yolks from birds fed VMP 5 had higher values (P<0.05) of TC, TG and VLDL but lower (P<0.05) HDL and LDL compared with others. This is because as vitamins in the diets were increased, there was increased vitamin deposition in the albumen and/or yolk [27]. Conversely, yolks from birds on VMP 1, 2, and 3 had similar values (p>0.05) of TC and TG but differing HDL, LDL and VLDL. Also of particular interest was the yolk of hens fed VMP 4 which recorded the lowest TC and TG mg/dL of 12.33 and 28.00 but had proportionately higher HDL compared to others. The observed effects of the different VMP did not follow a particular trend but it indicated that VMP was one of the main determinants of lipid fraction deposition in the egg yolk.
3.3. Effect of Interactions of RS and VMP on Egg Lipids
- The interactions of VMP and RS on TC was significant (p<0.05). Egg yolk from hens fed T5 had the highest TC of 16.67mg/dL which was significantly different (P<0.05) from those fed T4 12.33mg/dL while the interaction between BC x VMP 5 produced egg yolk with the highest TC value of 18.67mg/dL which was significantly higher (p<0.05) compared with others. This may be attributed to relatively higher levels of vitamins in T5. Increased vitamin E is known to influence TC, hence, high yolk TC could also be due to synergistic effect of BC housing system and the higher level of vitamin E, selenium, and vitamin B6 content in T5. It was reported [7] that eggs from free range chickens contained two thirds the amount of cholesterol compared to conventional cages. There were significant effects (P<0.05) of interactions between the five dietary VMP and the RS on all the lipid indices. Birds fed T5 had consistently higher (p<0.05) levels of TG in egg yolk with the value 65.67mg/dL compared with those from T1, T2, T3 and T4 with the corresponding values of 44.50, 48.67, 42.83, and 28.00mg/dL respectively. This was adduced to the vitamin-mineral composition of VMP 5.
3.4. Eggs Keeping Quality as Affected by RS and Dietary VMP
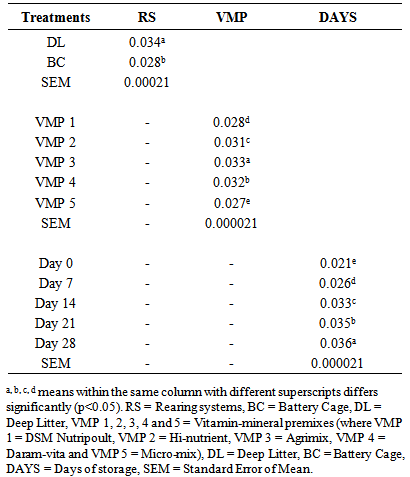
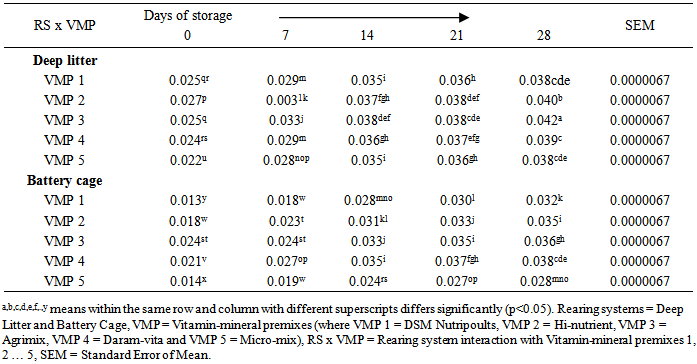
- Table 4 shows the keeping quality of eggs stored from 0 to 28 days in both RS as affected by dietary VMP. The TBARS is a measure of the presence MDA formed via lipid peroxidation. There were significant differences (p<0.05) in the values from both RS. The DL yolk recorded 0.034 against 0.028 recorded for BC. The observed increased oxidation of lipid in the yolk of eggs from DL was attributed to contamination of the egg by faeces, litter materials, bacteria and chemicals as earlier reported [4]. The corresponding TBARS of yolk obtained from hens fed T2, T3, T4 and T5 respectively were 0.031, 0.033, 0.032 and 0.027mg/dL. A significantly higher MDA in the egg yolk of hen on T3 was attributed to a lower level of vitamin E, vitamin B6 and selenium against birds on T5. Vitamin E protects cell from reactive oxygen species (ROS) [8]. Eggs from birds on T5 was observed to have delayed the deterioration of egg yolk while the synergistic interaction of BC x T5 slowed down lipid peroxidation up to 28 days of storage at room temperature. Each storage day differed from one another statistically with days 0 and 28 recording the lowest (0.021mg/g) and the highest (0.036mg/g) values respectively.
|
|
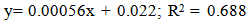 | (1) |
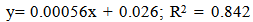 | (2) |
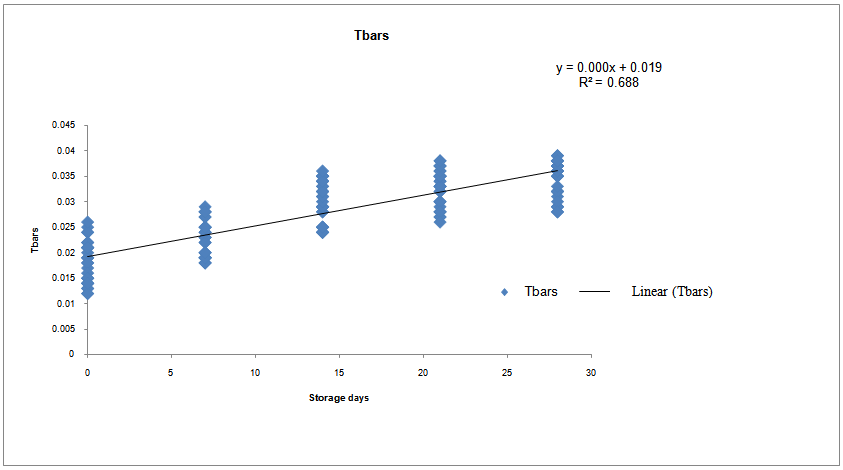 | Figure 1. Relationship of TBARS and duration of storage of eggs from Battery Cage rearing system |
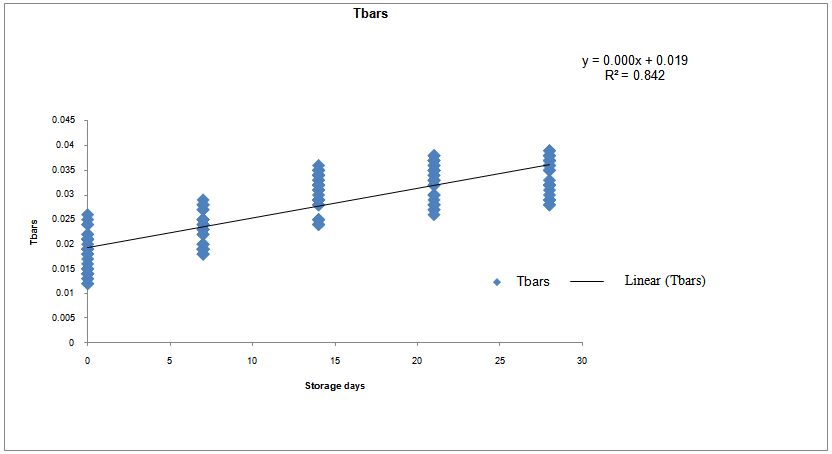 | Figure 2. Relationship of TBARS and duration of storage of eggs from Deep litter rearing system |
4. Conclusions
- Empirical findings from this experiment revealed that the lipid indices and duration of storage of eggs from both rearing systems were affected by the different dietary VMP. Also, the interactions of the dietary VMP and RS as well as DOS profoundly affected the lipid composition of eggs.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML