-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2015; 5(1): 1-9
doi:10.5923/j.fph.20150501.01
Effect of the Flavonol Quercetin on Human Platelet Function: A Review
Sapha Mosawy
Heart Foundation Research Centre, Griffith Health Institute, Griffith University, Gold Coast Campus, Qld, Australia
Correspondence to: Sapha Mosawy, Heart Foundation Research Centre, Griffith Health Institute, Griffith University, Gold Coast Campus, Qld, Australia.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Quercetin (Que) is one of the most abundant and potent naturally occurring antioxidant. Que has been shown to exert many biological activities, including antiplatelet activity. Indeed, Que was shown to inhibit platelet aggregation in response to platelet agonists, such as ADP, collagen, thrombin and arachidonic acid. However, the lowest Que concentration that significantly inhibits agonist-induced platelet aggregation remains contradictory. In addition, to anti-aggregatory effects, Que was demonstrated to inhibit platelet dense and alpha granule exocytosis when stimulated by different platelet agonists. Que was also shown to inhibit multiple platelet protein kinases, including, PI3K, Akt, PLC and PKC. The main aim of this review focuses on the inhibitory effects of Que on human platelet function.
Keywords: Flavonols, Que, Platelet, Thrombosis
Cite this paper: Sapha Mosawy, Effect of the Flavonol Quercetin on Human Platelet Function: A Review, Food and Public Health, Vol. 5 No. 1, 2015, pp. 1-9. doi: 10.5923/j.fph.20150501.01.
Article Outline
1. Introduction
- Platelets play an important role in the development of cardiovascular disease (CVD) and the formation of arterial thrombosis [1, 2]. Nitric oxide (NO) from the endothelial cells regulates platelet aggregation by increasing cyclic guanosine monophosphate (cGMP). However, in CVD, the endothelial cells are damaged and as a result the subendothelial matrix and collagen are exposed [3]. Platelet adhesion to subendothelial matrix is a crucial step in the formation of platelet rich thrombus. Platelet adhesion and activation is initiated by binding of collagen and von willibrand factor (vWF) to their platelet receptors. Following the initial adhesion and activation, platelets undergo further activation by releasing their granule content that include adenosine diphosphate (ADP) and formation of thromboxane A2 (TXA2) [4]. Platelet activation leads to a conformational change in platelet fibrinogen receptor GPIIbIIIa, which acts as a bridge between activated platelets, resulting in platelet aggregation [5]. Antithrombotic agents are regularly used for the secondary prevention of cardiovascular events in diabetic patients and in primary prevention of high risk individuals [6]. Furthermore, antiplatelet therapy is the standard treatment for patients undergoing percutaneous coronary intervention (PCI). Despite the clinical benefits achieved with antiplatelet therapy, some patients still develop thrombotic episodes [7, 8], and there is growing data to suggest inadequate cardiovascular protection by these agents [9].Epidemiological studies have suggested that consumption of a flavonol rich diet is associated with reduced mortality due to CVD [10]. Indeed, the Rotterdam study showed reduction in the occurrence of fatal myocardial infarction with flavonol consumption [11]. Furthermore, flavonols have been shown to exert both antioxidant and antiplatelet activity in vitro [12-14]. Rechner et al. [15] had shown that dietary polyphenolic compounds inhibit platelet aggregation, and Hubbard et al. [16] reported that following ingestion of the naturally occurring flavonol Que, collagen-induced platelet aggregation was inhibited. In addition, previous studies have shown that ingestion of flavonol rich foods and beverages reduce platelet aggregation induced by different agonists [17-19]. The present review focuses on the effect of the flavonol Que on human platelet function.
2. Flavonols
- Flavonols are a subgroup of flavonoids. Flavonoids are low molecular weight phenolic substances widely found in plants, fruits and vegetables [12, 20]. Flavonols are well known to be potent scavengers of free radicals. This important biological activity is principally based on the redox properties of their phenolic hydroxyl groups, and the structural relationships between different parts of their chemical structure. Flavonols have three structural groups that determine their antioxidant activity, which are: the o-dihydroxy (catechol) structure in the B-ring, the 2, 3-double bond conjugated with a 4- oxo function and the presence of hydroxyl (OH) groups. These structural features and particularly the number of OH groups present are uniquely arranged giving different flavonols varying antioxidant capacity [21, 22]. Flavonols have been shown to exert different biological activities. These include antioxidant, antimicrobial, antiviral and antiplatelet activities [12-14].
2.1. Quercetin
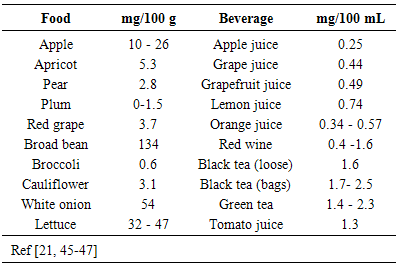
- Que (3,5,7,3’,4’-pentahydroxyflavone) (Fig 1) is one of the most abundant flavonols, and due to its chemical structure is one of the most potent naturally occurring antioxidants within the flavonoid subclasses. It is ubiquitously found in a variety of fruits, vegetables and plant derived beverages [21]. Table 1 shows Que concentration in different pants and plant- derived beverages. In food, Que is lipophilic, and is mainly bound to sugars, phenolic acids or alcohols [23]. However, following ingestion, Que and its derivatives are hydrolysed mostly in the gastrointestinal tract, then absorbed and metabolised, Que absorption rate depends on the food source being ingested. The absorbed forms of Que are mainly glucosides and aglycones [21]. In addition to Que antioxidant and cardiovascular beneficial properties, it has been was found to produce several other important biological effects, including anti-inflammatory, antiplatelet and anti-hypertensive effects [24, 25].
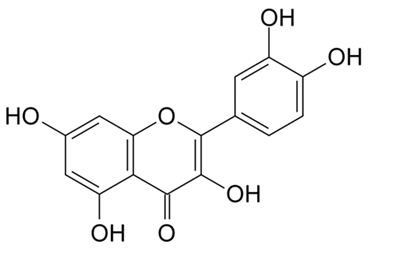 | Figure 1. Molecular structure of Que |
|
3. Platelet and Arterial Thrombosis
- It is well established that platelets play an essential role in the formation of arterial thrombosis and vascular complications [26]. Platelets from vascular disease patients were found to be hyperactive, and there were increased number of circulating monocyte-platelet aggregates [27, 28]. In addition to their high sensitivity to agonists, platelet secrete chemokines and cytokines that attract monocytes and neutrophils [29], hence further contributing to the ongoing inflammatory processes at the vessel wall, accelerating the progression of atherosclerosis and development of CVD [30, 31]. Injury to the vascular endothelial lining exposes the highly thrombogenic subendothelial matrix on which collagen and vWF are found [32, 33], the damaged vascular wall releases tissue factor, while coagulation factor VIIa is released from platelet alpha granules initiating the coagulation cascade [34, 35]. Collagen and vWF bind to their specific platelet receptors causing platelet tethering and adhesion to the damaged vessel wall leading to a platelet rich thrombus.
3.1. Antiplatelet Therapy
- Aspirin has been the drug of choice for patients with cardiovascular disease or as a prophylaxis for many years, because it is cost effective and readily available [36, 37]. Aspirin inhibits platelet function via the irreversible acetylation of COX 1, by inactivating its catalytic activity. COX 1 catalyses the formation of arachidonic acid to prostaglandin H2, and finally TXA2 formation [38]. Inhibition of TXA2 results in the blockade of platelet activation through the thromboxane receptor.Although aspirin has been the most common antiplatelet agent for many decades, there are some limitations and concerns associated with it that must be taken into consideration. It has been shown that that aspirin is a weak platelet inhibitor [8, 39]. Furthermore, CVD patients on aspirin are becoming less responsive to aspirin [40, 41], where patients with CVD have recurrent thrombotic events while on aspirin therapy [38, 42]. Therefore, many of such patients are placed on dual antiplatelet therapy such as aspirin and clopidogrel [43]. Clopidogrel and ticlopidine (ADP receptor antagonists) [36] have been shown to be effective antiplatelet agents. However, there is an increasing body of evidence suggesting increased non responsiveness to clopidogrel amongst cardiovascular patients on a standard dose [41, 44].
4. Anti-platelet Properties of Que
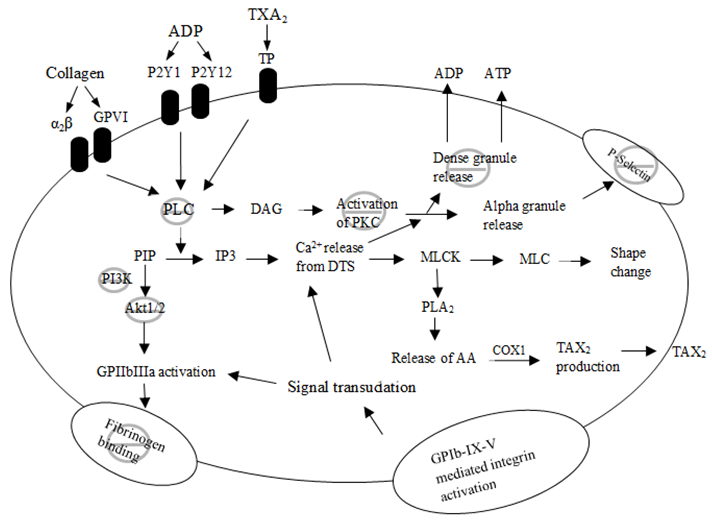
- Platelet aggregation is the final step in the thrombus formation process. Platelet aggregation is stimulated by different agonists via different pathways. The effect of these agonists is mediated by an increase in intracellular calcium concentration, which induces the activation of different enzymes such as myosin light chain kinase (MLCK) and calcium – dependant phospholipase A2. The activation of these enzymes leads to phosphorylation of actin filaments and induces platelet shape change. Following shape change platelet undergo release reaction in which platelets’ dense and alpha granules release their content which results in an amplification in the activation process [48, 49].
4.1. Effect on Platelet Aggregation
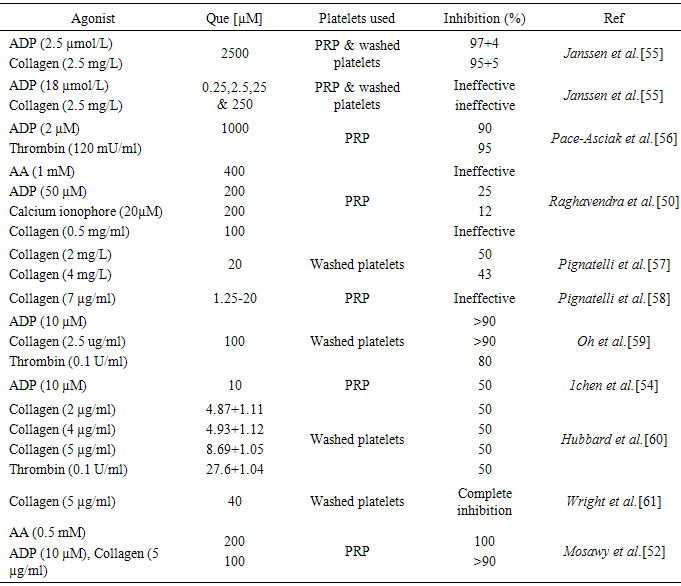
- While Que has been demonstrated to reduce or inhibit platelet aggregation, the degree of inhibition and the effective Que concentrations are varying. Indeed, some studies have shown Que to be very effective in reducing platelet aggregation at low concentrations, while other studies have reported higher concentrations were required to achieve anti-aggregatory affects. Beretz et al. [13] reported inhibition of collagen induced platelet aggregation with an IC50 of 55 µM, while Raghavendra et al. [50] reported 100 µM of Que caused only 1.7% inhibition of collagen-induced aggregation. In a study by Sheu et al. [51] demonstrating the inhibition of collagen-induced platelet aggregation by Rutin (Que glycoside) at 250 and 290 µM. We have demonstrated that Que at 100 µM caused >90% inhibition of collagen-induced platelet aggregation [52]. Que was also demonstrated to have variable and conflicting effects on platelet aggregation induced by other platelet agonists. Landolfi et al. [53] reported inhibition of arachidonic acid (AA) induced platelet aggregation by Que with an IC50 of 18 µM, while we showed complete inhibition of arachidonic acid-induced platelet aggregation by Que at 200 µM [52]. In a different study by Raghavendra et al. [50] suggested that Que was ineffective inhibitor of AA induced platelet aggregation with an IC50 of >400 µM. Table 2 summarises the reported effect of Que on platelet aggregation induced by different agonists.
|
4.2. Effect on Platelet Signalling Proteins
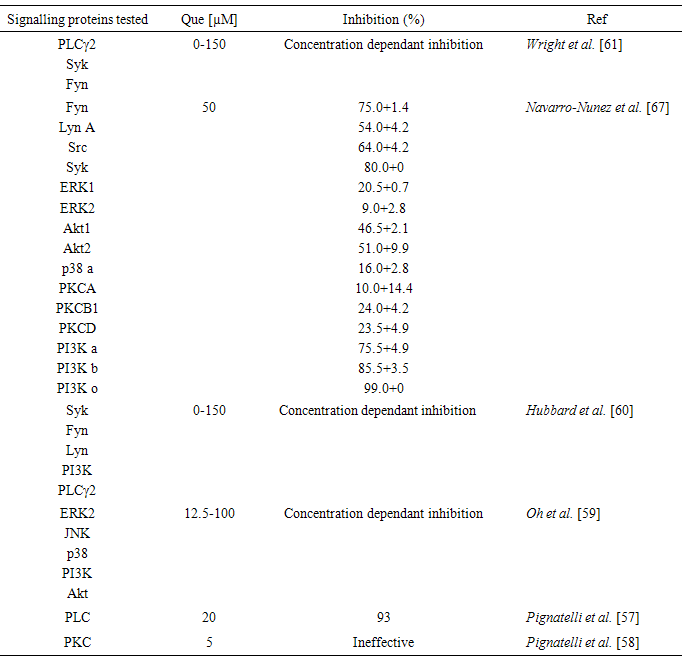
- Platelet signalling proteins play a major role in platelet activation and aggregation. Binding of fibrinogen or vWF to platelet αIIbβ3 transmits signals that regulate the extension of platelet filopodia, spreading, granule release and aggregation. The protein kinase Syk is involved in the initiation of αIIbβ3 – dependant actin polymerization and platelet spreading. While the protein kinase Src is needed for αIIbβ3 – dependent tyrosine phosphorylation of Syk and for platelet spreading on fibrinogen [62, 63]. It is reported that c-Src and Lyn kinases form complexes with phosphoinositide 3-kinase (PI3K) and GP1b-IX-V after vWF stimulation to induce platelet activation. Limited number of studies have attempted to investigate the effect of Que on several signalling proteins, Navarro-Núñez et al. [64] tested the effect of 50 µM Que on different platelet signalling proteins, the authors reported significant inhibition of Fyn, Lyn, Src and Syk protein kinases. While, Hubbard et al. [60] and Wright et al. [61], reported Que at 0-150 µM inhibited Fyn and Syk protein kinases in a concentration dependant manner.It has been suggested that PI3K plays an important role in GP1b-IX-V mediated, integrin – dependant platelet adhesion, spreading and aggregation. Navarro-Núñez et al found that Que at 50 µM completely inhibited all PI3k isoforms. In a different study, Agullo et al. [65] demonstrated Que at 60 µM caused near complete inhibition of PI3k alpha. One of PI3K functions is to phosphorylate and activate Akt 1 and Akt 2 via phosphoinositide dependant protein kinase (PDK). Akt kinases have been found to mediate GPIb-IX signalling via G-coupled protein signalling pathways, which leads to the amplification of platelet aggregation induced by G-protein coupled receptors and collagen [66]. Several studies have reported inhibition of Akt by Que [59, 67].Protein kinase C (PKC) is expressed in human platelets and it has been found to play an important role in many platelet signalling pathways [68]. It has also been suggested that the phosphorylation of PKC regulates dense granule release mediated by PAR platelet receptors. Que has been found to weakly inhibited PKC isoforms [58]. Table 3 shows the effect of Que on platelet signalling proteins.
|
4.3. Effect on other Platelet Function Parameters
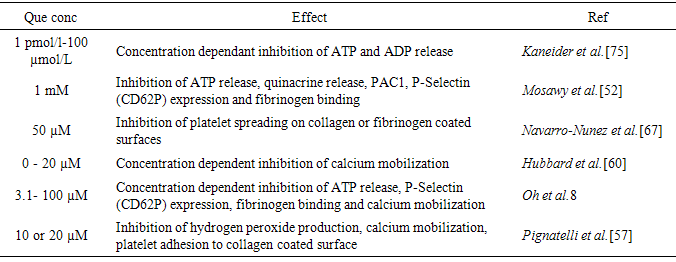
- Platelet activation and aggregation is a complex and multi-step process. Platelet dense granules play an important role in the amplification of platelet activation by the release of signalling and activation molecules such as ATP and ADP, while the content of platelet alpha granules are essential for platelet adhesion to endothelial cells and leucocytes [69]. Que has been demonstrated to inhibit the exocytosis of both dense and alpha granules, calcium mobilization and platelet spreading [52, 59, 67]. Table 4 summarises Que effect on platelet granule exocytosis and other activation parameters.
|
5. Effect of Que Metabolites on Platelet Function
- In food, Que is lipophilic, and is mainly bound to sugars, phenolic acids or alcohols [70]. However, following ingestion, Que is hydrolysed, and then absorbed and metabolised, Que absorption rate depends on the food source being ingested. The absorbed forms of Que are mainly glucosides and aglycones [21]. There are many different quercetin-conjugated metabolites have been identified [71], the main conjugates were found to be tamarixetin, quercetin-3’-sulphate, quercetin-3’glucuronide and Que-4’-O-B-D-glucoside [71]. Many studies have explored the antioxidant activities of many of these conjugates [72-74]. However, the antiplatelet potential of quercetin conjugates has not been widely investigated. Nevertheless, there is a limited number of studies that have attempted to elucidate the antiplatelet activity of some quercetin conjugates. Indeed, Hubbard et al. [16] reported that Que-4’-O-B-D-glucoside was able to inhibit collagen-induced platelet activation. It was demonstrated that ingestion 150 or 300 mg of Que-4’-O-B-D-glucoside inhibited platelet aggregation, tyrosine phosphorylation of SyK and PLCγ2. In a different study by Wright et al. [61] investigating the effects of the metabolites tamarixetin, Que-3’-sulphate and Que-3’-glucuronide on platelet function. It was found that tamarixetin and Que-3’-sulphate completely inhibited platelet aggregation, whereas, Que-3’glucuronide did not cause significant inhibition of platelet aggregation at the same concentration. Wright el al. [61] also demonstrated that neither of these metabolites was able to significantly inhibited Fyn kinase, whereas, tamarixetin, but not Que-3’-sulphate or Que-3’glucuronide, was able to significantly inhibit total protein phosphorylation, Syk and PLCγ2 tyrosine phosphorylation. These data suggest structural modification of Que following ingestion decreases its potency to inhibit platelet function.
6. Conclusions
- It is well established that platelets are essential in the formation of arterial thrombosis and development of CVD. Therefore, antiplatelet is the mainstay treatment for patients who are at high risk of developing thrombotic episodes. However, increasing evidence has indicated inadequate protection by antiplatelet therapy against thrombotic events in some patients [8]. Several studies have therefore investigated many polyphenolic compounds for their cardiovascular and antiplatelet potentials. Que is a polyphenolic flavonol that is been found to be a potent antioxidant with many beneficial activates that include antiplatelet activity. Indeed, Que and some of its metabolites have been demonstrated to inhibit platelet aggregation and activation. However, there are conflicting data regarding the lowest Que concentration capable of producing significant antiplatelet effect and whether they are achievable in vivo. Furthermore, the exact mechanism/s by which platelet function is inhibited is not fully understood. Several studies have shown inhibition of multiple platelet signalling proteins, while others demonstrated inhibition of platelet granule exocytosis. It is likely that Que inhibits platelet function via multiple mechanisms, and at least in part due to, the inhibition of signalling molecules and granule exocytosis (Fig 2).This review identifies several limitations associated with currently available data, including, the lowest effective Que concentration and agonist concentration. An effective strategy to overcome the agonist concentration issue is to construct a dose-dependent curve for the agonist concentrations, and then appropriately choosing the effective concentration.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML