-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2014; 4(6): 286-292
doi:10.5923/j.fph.20140406.05
Assessment of Proximate Chemical Composition, Nutritional Status, Fatty Acid Composition and Antioxidants of Curcumin (Zingiberaceae) and Mustard Seeds Powders (Brassicaceae)
M. Kamal E. Youssef1, Amira M. El Newihi2, Soad M. Omar3, Zeinab S. Ahmed3
1Food Science and Technology Department, Faculty of Agri., Assiut University, Assiut University, Assiut, Egypt
2Medical Biochemistry Department, Faculty of Medicine, Assuit University, Assuit, Egypt
3Home Economic Department (Nutrition and Food Science), Faculty of Specific Education, Assuit University, Assuit, Egypt
Correspondence to: Zeinab S. Ahmed, Home Economic Department (Nutrition and Food Science), Faculty of Specific Education, Assuit University, Assuit, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Curcumin is a spice grown in India, which belongs to Zingiberaceae family. Mustard seeds plant belonging to brassica family which also includes cabbage, mustards are cultivated in Canada, India, China. curcumin powder had a high content of total carbohydrates (67.91%) and low contents of fat (2.46%), fiber (4.02%) and protein (9.34%). curcumin powder is rich source of Ca, P, K and Mg as well as total phenols, flavonoids and vitamin C. Mustard seeds powder had a high content of fat (43.85%), protein (25.81%) and fiber (7.00 %) and low contents of total carbohydrates (16.38%). It is rich source of Fe, Mg and Na as well as flavonoids, lycopene and vitamin E. Curcumin and mustard seeds powders oil consisted mainly of Oleic and Linoleic acid recording ( 0.7 and 0.9g/100gm) and ( 0.9 and 0.8g/100gm); respectively. Curcumin and mustard seeds powders consisted amino acids mainly of lysine (0.05 and 0.08g/100gm protein). Curcumin and mustard seeds powders had high nutritional value due to its high dietary fiber and antioxidants compounds. The soluble fibers exert a preventative role against heart disease and lowering serum cholesterol.
Keywords: Curcumin, Mustard seeds powder, Chemical composition, Minerals, Amino acids, Fatty acids, Antioxidants
Cite this paper: M. Kamal E. Youssef, Amira M. El Newihi, Soad M. Omar, Zeinab S. Ahmed, Assessment of Proximate Chemical Composition, Nutritional Status, Fatty Acid Composition and Antioxidants of Curcumin (Zingiberaceae) and Mustard Seeds Powders (Brassicaceae), Food and Public Health, Vol. 4 No. 6, 2014, pp. 286-292. doi: 10.5923/j.fph.20140406.05.
Article Outline
1. Introduction
- Curcumin is a spice grown in India and other tropical regions of Asia, which belongs to Zingiberaceae family in India [1]. It had a long history of use in herbal remedies, particularly in China, India, and Indonesia. The root and rootstock, or rhizome, of the plant contains the active ingredient [2]. Although the ability of curcumin to preserve food through its antioxidant mechanism, to give color to food, and to add taste to the food is well known [3].Mustard seeds had been highly prized medicinal as well as culinary spice being in use since ancient times. The seeds are obtained from mustard plant belonging to brassica family which also includes cabbage, mustards are native of Asia minor, but now cultivated as a main commercial crop in Canada, India, China, and temperate climates of European region [4]. Specialty mustards, which include almost every possible blend of added flavors and range of textures. The use of mustard in restaurants and in home cooking has expanded and become more subtle and more adventurous [5]. Curcumin and mustard seeds contain protein, fat, minerals, carbohydrates, moisture, oils, sugars, vitamins, phyto-nutrients and antioxidants [6].The chemical composition of curcumin was studied by [7]. Curcumin contains protein (6.3%), fat (5.1%), minerals (3.5%), carbohydrates (69.4%) and moisture (13.1%). The essential oil (5.8%) obtained by steam distillation of rhizomes.Mustard seeds contain protein (24.9%), crude fiber (14.7%), carbohydrates (34.94%), sugar (6.89%) [8]. Mustard seeds contain protein (26.08%), crude fiber (12.2%), carbohydrates (28.09%), fat (33.63%) on dry weight basis [9].100g mustard seeds contained 3.2 % moisture, 31.70 g protein, 42.60g fats, 18.50 g total carbohydrates 1.90 g fiber on dry weight basis [10]. Mustard seeds powder contained about 34-39% protein [11]. Mustard seeds contained 8.02 % moisture, 32.4 protein, 1.5 % ash, 2.7% fiber and 31.72 % total carbohydrates on dry weight basis [12].The mustard seeds had high content in essential oils. The seeds had high in calories; 100 g of seeds contain 508 calories. However, they had good source of dietary fiber; so they are recommended in cholesterol controlling and weight reduction programs [13]. Their composition depends on the variety, climate and growing technigues.Curcumin powder is characterized by relatively hot taste which is due to aromatic odor of curcumin because it contains essential oils that can be easily separated from the rest of the ingredients by steam distillation. The food industry uses curcumin to give color in butter, cheese and cooked rice [1]. Curcumin is the product obtained by solvent extraction of turmeric, the ground rhizomes of curcuma longa and purification of the extract by crystallization. Curcumin is an orange – yellow crystalline powder. Minor amounts of oils and resins naturally occurring in turmeric may be present [14].Curcumin and mustard seeds are an excellent source of phenolic compounds (flavonoids, phenolic acid and alcohols, stilbenes, tocopherols, tocotrienols), ascorbic acid and carotenoids which had been reported to show good antioxidant activity [15]. These curcuminoids are known to have high antioxidant activites [16]. Curcumin, a poly-phenolic compound, is the principal pigment that imparts deep orange color to the turmeric. Polyphenols appear as one of the most promising groups in plants. Polyphenols are important for growth and protection against pathogens. Polyphenols had recently received much attention in disease prevention and treatment due to their proven antioxidants capabilities [17].Oil mustard seeds had been shown to impart several beneficial effects of which the antioxidant effect is most pronounced [18]. It had been found that mustard had higher antioxidant activity as compared to fruits, cereals and nuts. The active components in spices phthalides, polyacetylones, phenolic acids, flavonoids, coumarins and terpenes were reported as powerful antioxidants [19]. Mustard had not only been found to be effective antioxidant in vivo and vitro to deal with oxidation stresses but also quite active in stabilizing the edible oils and fatty food against rancidity and oxidative deterioration [20]. Studies had suggested that preventive or putative therapeutic properties of curcumin [21] and mustard seeds [22] had also been considered to be associated with their antioxidant property. Because free radical-mediated peroxidation of membrane lipids and oxidative damage of DNA and proteins are believed to be associated with a variety of chronic pathological complications such as cancer, atherosclerosis, neurodegenerative diseases, and aging, [23] curcumin and mustard seeds are thought to play a vital role against oxidative-stress-mediated pathological conditions.Curcumin [24] and mustard seeds [25] have a wide spectrum of biological actions, including its anti-inflammatory, antioxidant, anticarcinogenic,antimutagenic, anticoagulant, antifertility, antidiabetic, antibacterial, antifungal, antiprotozoal, antiviral, antifibrotic, antivenom, antiulcer, hypotensive and hypocholesteremic activities. The present investigation was carried out in an attempt to clarify the proximate chemical composition, the nutritional status, as well as, the fatty acid composition, amino acid composition and antioxidants of curcumin and mustard seeds powders.
2. Materials and Methods
2.1. Materials
- Two Kg of both curcumin and mustard seeds were obtained from Medical and Aromatic Plants Department, Agriculture Research center, Giza, Egypt. Season 2012.
2.2. Methods
2.2.1. Determination of Gross Chemical Composition
- Crude Fat, fiber, protein, moisture, ash content were determined according to the procedures described in the AOAC [26]. The total carbohydrates were calculated by difference according to [27]. Caloric value was calculated according to [28].
2.2.2. Determination of Mineral Contents
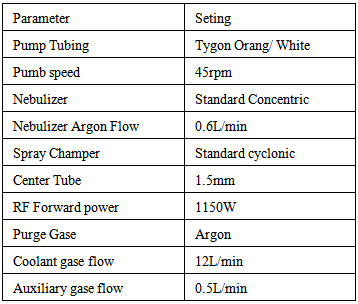
- Total content of elements- was carried out using a mixture of (HClO4/HNO3) according to (Inductivelv Coupled Plasma Emission Spectrometry) The elements (Ca, Mg, Mn, Cu and Fe) were determined using the ICP (iCAP620) according to [29] Standard preparation:Multi-element working standard were prepared by dilution of the stock standards with mixed acid blank solution.
|
2.2.3. Determination of Antioxidants
2.2.3.1. Determination of Phenols Content
- Phenols were determined colorimetrically using (model 8341) as described by [31]. Sample (5g) was mixed with 50 ml 80% ethanol in a dark bottle at 5 C for 72 h. Extracts were obtained by changing the ethanol every 24 h. The extracts were collected after filtration and the color developed by Folin-Ciocalteau reagents (FCR) was measured spectrophotometrically at 760ηm using a Spectrophotometer (Shimadzu Corporation, 2011, Kyoto, Japan).
2.2.3.2. Determination of Total Flavonoids Content
- Flavonoids were determined according to the method of [32]. The flavonoids in ethanol extract were estimated using aluminum chloride reagent. Five ml of the extract were added in test tube then 3 ml (aluminum chloride, 2.4%) and potassium acetate (9.8%) were added. After 5 min, the yellow color was measured at 760 ηm against blank reagent using a Spectrophotometer (Shimadzu Corporation, 2011, Kyoto, Japan).
2.2.3.3. Determination of Lycopene
- Lycopene from samples were extracted with hexane, methanol, acetone (2:1:1), containing 2.5% BHT. The extract was treated with doubly distilled water, methanol and 20% KOH/methanol (1:1:1) to saponify any triglyceride present. The extract is then washed with doubly distilled water and re-dissolved in hexane. Optical density of the hexane extract was measured spectrophotometrically (Shimadzu Corporation, 2011, Kyoto, Japan) at the wavelength 400-750 nm against hexane as blank. Concentrations of lycopene and β-carotene were calculated at λ max 505 and 487 ηm respectively [33].
2.2.3.4. Determination of Vitamins (C and E)
- The most satisfactory chemical methods for estimating ascorbic acid based on the reduction of 2, 6-dichlorophenol indophenols by ascorbic acid [34] was used for determining ascorbic acid in samples.Vitamin E determination was carried out on an aliquot by measuring absorbance at 470 ηm and 270 ηm respectively, in a Genesis-5 Spectronic spec- trophotometer (Rochester, NY) against a blank sample (solvent) [34].
2.2.4. Determination of Amino Acids Content of Curcumin and Mustard Seeds Samples
- Sample of 50-100 mg of dried curcumin and mustard seeds powder were weighed in the screw- capped tubes and 5 ml of (6.0 N) HCL were added. The hydrolysis tubes were attached to a system, which allowed the connection of nitrogen and vacuum lines without disturbing the sample. The tubes were placed in an oven at 110 C for 24 h. [30]. The tubes were then opened and the content of each tube was then filtered and evaporated for dryness in a rotary evaporator. A suitable volume of sodium citrate buffer (ρH 2.2) was added to each dried film of the hydrolyzed sample. After all soluble materials were completely dissolved the sample was then filtered using a 0.2 µm membrance filter, the samples were ready for analysis [35].The system used for the analysis was High Performance amino Acid Analyzer, Biochrom 20 (Auto sampler version) Pharmacia Biotch constructed at NCRRT. Data analysis of chromatogram was done by EZChrom Chromatography Data System Tutorial and users Guide-Version 6.7. Tryptophan was determined by the colormeteric method using Uv-1601Pc, SHIMADZU, UV-VISBLE spectrophotometer (550 ηm) according to the method described by [36].
2.2.5. Determination of Fatty Acids Methyl Esters by Gas Liquid Chromatography
- The methyl esters of fatty acids were separated using a PYE Unicam pro-GC gas liquid chromatography with a dual flame ionization and carried out on (3.0 m × 0.25 mm) SP-2310 column, packed with 55% cyanopropyl phenyl silicone dimensions. Column temperature: At first the temperature was 100℃ at the rate of 8 ℃/ minute, and then isothermal for 10 minutes at 195℃. The injector and detector temperature were 250℃ and 300℃; respectively.Carrier gas: Nitrogen at the rate 30 ml / minute. Hydrogen flow rate were 33 ml / minute and air flow rate 330 ml/ minute. The chart speed was 0.4 cm/ minute. Peak identifications were established by comparing the retention times obtained with standard methyl esters. The areas under the chromatographic peak were measured with an electronic integrator [37].
3. Results and Discussion
3.1. Gross Chemical Composition and Caloric Value
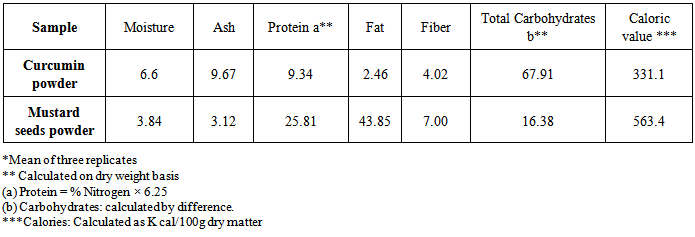
- Spices and herbs had been used for thousands of centuries by many cultures to enhance the flavor and aroma of foods. Early cultures also recognized the value of using spices and herbs in preserving foods and for their medicinal value [38].The data of gross chemical composition and caloric value of curcumin and mustard seeds powders are presented in table (1).
|
3.2. Minerals Content
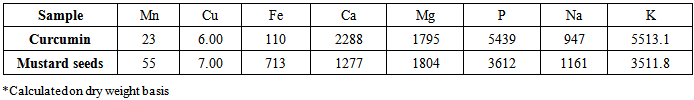
- The data of the average values of minerals content in curcumin and mustard seeds powders are outlined in Table (2).
|
3.3. Antioxidants Content
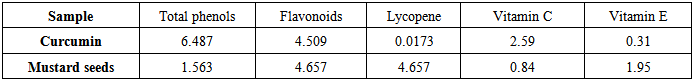
- The data outlined in Table (3) represented the mean values of antioxidants content in curcumin and mustard seed powders.
|
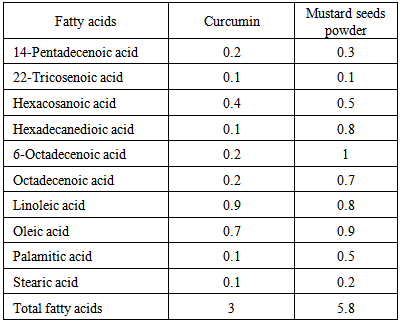
3.4. Fatty Acid Composition
- The fatty acids composition data of curcumin and mustard seeds powders are presented in Table (4). The obtained data revealed that curcumin powder had highest amounts of Linoleic and Oleic acid (0.9 and 0.7g/100g). In contrary, it had lowest amounts of Stearic, Palamitic, Hexadecanedioic and 22-Tricosenoic acid (0.1g/100g), 6-Octadecenoic, Octadecenoic and 14-Pentadecenoic acid (0.2 g/100g); respectively. The data are in good agreement with [49]. While mustard seeds powder contained the highest fatty acids were 6-Octadecenoic, Oleic, Linoleic, Hexadecanedioic acid (1, 0.9, 0.8 and 0.8 g/ 100g); respectively. The data are in good accordance with [50]. In contrary, it had lowest values of fatty acids were recorded for 22-Tricosenoic, Stearic and 14-Pentadecenoic acid (0.1, 0.2 and 0.3 g/100g); respectively. Such results are in accordance with [51, 52].
|
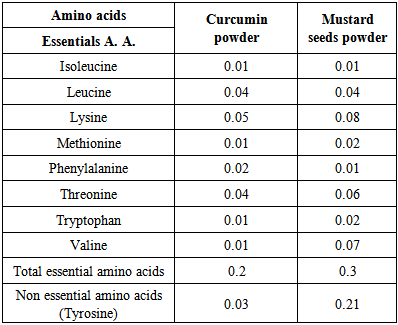
3.5. Amino Acids Content
- The amino acids composition data of curcumin and mustard seeds powders are presented in Table (5). The obtained data revealed that curcumin powder had highest amounts of lysine (0.05g / 100g), in contrary, it had lowest amounts of Isoleucine, Methionine, Tryptophan and valine (0.01g / 100g). These results are in accordance with [53]. While, mustard seeds powders had highest essential amino acids were tyrosine, lysine and valine (0.21, 0.08 and 0.07 g / 100g); respectively. The date are in agreement with [50]. In contrary, the least values of essential amino acids in mustard seeds powder were recorded Isoleucine and Phenylalanine (0.01g /100g). These results are in accordance with [54, 55].
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML