-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2014; 4(6): 272-278
doi:10.5923/j.fph.20140406.03
Allicin-Rich Extract Obtained from Garlic by Pressurized Liquid Extraction: Quantitative Determination of Allicin in Garlic Samples
Angela M. Farías-Campomanes1, Claudia N. Horita2, Marise A. R. Pollonio2, M. Angela A. Meireles1
1LASEFI/DEA (Department of Food Engineering)/FEA (School of Food Engineering)/UNICAMP (University of Campinas), Rua Monteiro Lobato, Campinas, Brazil
2DTA (Department of Food Technology)/ FEA (School of Food Engineering)/ UNICAMP (University of Campinas), Rua Monteiro Lobato, Campinas, Brazil
Correspondence to: M. Angela A. Meireles, LASEFI/DEA (Department of Food Engineering)/FEA (School of Food Engineering)/UNICAMP (University of Campinas), Rua Monteiro Lobato, Campinas, Brazil.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Since ancient times, garlic has been used to prevent and treat various diseases. The health benefits of garlic are attributed to its content of thiosulfinates, of which allicin is the main bioactive compound. Allicin is not found in fresh garlic; it is derived from alliin through the enzymatic action of alliinase when the membranes of the garlic cloves are destroyed. Allicin is a very unstable compound, degrading within a few minutes of being produced, at high temperatures and in the presence of certain solvents. Pressurized liquid extraction (PLE) is a relatively new technique that has demonstrated high efficiency on the extraction of bioactive compounds. Thus, PLE of garlic at 313 K and 6 MPa, using ethanol as solvent, was performed. The concentration of allicin in the garlic PLE extract, garlic powder, garlic oil and fresh garlic was quantified using the Internal Standard method. The global yield of garlic PLE was 1.3 ± 0.3% on a wet basis. The garlic PLE extract had the highest allicin concentration (332 ± 5 µg of allicin.g-1 of sample), followed by fresh garlic and garlic powder. Allicin was not detected in garlic oil.
Keywords: Pressurized Liquid Extraction, Ethanol, Allicin, Garlic, Allium sativum L.
Cite this paper: Angela M. Farías-Campomanes, Claudia N. Horita, Marise A. R. Pollonio, M. Angela A. Meireles, Allicin-Rich Extract Obtained from Garlic by Pressurized Liquid Extraction: Quantitative Determination of Allicin in Garlic Samples, Food and Public Health, Vol. 4 No. 6, 2014, pp. 272-278. doi: 10.5923/j.fph.20140406.03.
Article Outline
1. Introduction
- Garlic (Allium sativum L.) and its derived products have been widely used for culinary and medicinal purposes by many cultures. Research has demonstrated that garlic has a wide range of biological activities, including antihypertensive, lipid-lowering, antibacterial, antifungal, and antiviral activities, among others [1]. Studies have shown a relationship between a high intake of garlic and a low risk of certain cancers [2-4]. The health benefits of garlic have been attributed to its thiosulfinates content. Allicin is the most prevalent of these bioactive compounds, representing approximately 70% of the thiosulfinates in garlic [5].Allicin was first reported in 1944 by Cavallito and Bailey [6], who described it as a colorless oil with low solubility in water that was relatively unstable. Allicin derives from the precursor alliin through the enzymatic activity of alliinase (Figure 1). According to Murray [7], 4 g of fresh garlic contains approximately 10 mg de alliin and can be converted into at least 4 mg of allicin. Alliin and alliinase are located in separate regions of garlic cloves, and the alliinase reaction is initiated only after the cells have been crushed [8, 9]. Allicin is completely formed in 0.3 min at 310 K [10] and, at room temperature, its half-life ranges from 10 days to few hours depending in the solvent in which it is dissolved [11]. Despite allicin being a low-polarity molecule, it is often extracted using polar solvents, such as water and ethanol, at room pressure (0.1 MPa) because it is very unstable in non-polar organic solvents [9]. Fujisawa et al. [12] concluded that vegetable oil and n-hexane are solvents that provide low extraction yields of allicin, most likely due to its high level of instability. Moreover, the temperature and concentration at which allicin is stored are factors that must be considered to prevent its degradation [12-14].The composition of thiosulfinates compounds in garlic products depends on the processing conditions [15]. According to Shi [16], spray-drying, freeze-drying and oven-drying at high temperature, of fresh garlic; result in a loss of activity or destroying of the alliinase, thus preventing allicin production. In contrast, over drying garlic at low temperature (< 333 K) has little infect on the yield of the allicin and other thiosulfinates. Additionally, garlic products in the form of oils do not generate allicin, but rather, allicin-derived compounds [16].
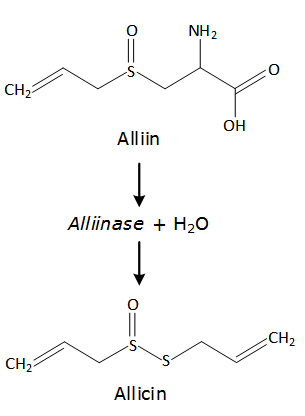 | Figure 1. Formation of allicin in fresh garlic |
2. Materials and Methods
2.1. Plant Material
- Fresh garlic was obtained at local supermarkets (Campinas, SP, Brazil). The outer skin of the garlic cloves was peeled off. The garlic cloves were cut into small cubes (approximately 0.3 cm on all side) using a kitchen knife. The garlic samples were prepared immediately before PLE extraction was performed in order to avoid the degradation of allicin.
2.2. Pressurized Liquid Extraction
- The PLE assays were performed using the home-made PLE system previously described by Rodrigues et al. [18], which consisted of an HPLC pump (Thermo Separations Products, model 3200 ConstaMetric P/F, Fremont, CA, USA), a manometer, an extraction vessel (Thar Designs, CL 1373, Pittsburg, PA, USA) that was heated using an electrical heating jacket and blocking and backpressure valves (Figure 2).A 6.3 cm3 (2.0 cm diameter and 2.0 cm height; internal dimensions) extraction vessel was completely filled with 6.6 ± 0.1 g of fresh garlic. The extraction assays were performed at 313 K and 6 MPa using a static extraction period of 5 min, in triplicate. Ethanol (99.5% purity, Dinamica, Campinas, Brazil) flowing at a rate of 2.6 × 10-5 kg.s-1 was used as the solvent, and the solvent mass to feed mass ratio (S/F) was maintained at 1.2. Ethanol was removed from the extracts by vacuum evaporation (Laborota, model 4001, Viertrieb, Germany) at 313 K and 0.1 MPa. The global extraction yield (X0, S/F) was calculated as the percentage (%) of the mass of the extract (mExtract) relative to the total mass of the raw material on a wet basis (mRM) that was used to perform the bed extraction, using Equation 1, as follows:
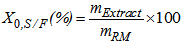 | (1) |
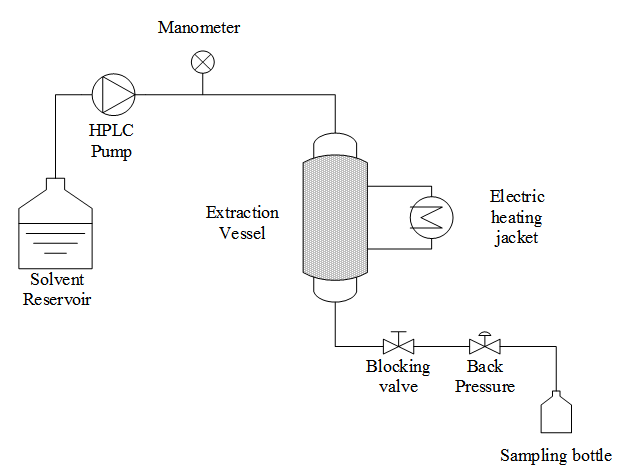 | Figure 2. Flow diagram of the home-made PLE unit used in this study |
2.3. Determination of the Allicin Concentration in the Garlic Samples
- The concentration of allicin in the garlic samples was determined using the Internal Standard method [8]. The internal standard used in this study was ethyl p-hydroxybenzoate, which was mixed with the garlic samples prior to performing the HPLC analysis. The internal standard must have a known concentration, a structure similar to that of allicin and must not react with it.
2.3.1. Preparation of the Internal Standard Solution
- Two internal standard solutions of different concentrations were prepared. First, an internal standard solution with a concentration of 0.5 mg.cm-3 was prepared. For this, approximately 200 mg of ethyl p-hydroxybenzoate (99%, lot P500011, Fluka) was added to 8 cm3 of methanol and the solution was shaken until it dissolved. Then, 360 cm3 of Milli-Q water at 353 K was added. Finally, 32 cm3 of Milli-Q water at room temperature was added and the solution was shaken. After performing the first HPLC analysis, it was observed that the concentration of this internal standard solution was much higher than the allicin concentration of the samples. Thus, to ensure the accuracy of the results, a second internal standard solution, with a concentration of 0.2 mg.cm-3, was prepared.
2.3.2. Preparation of Garlic Samples
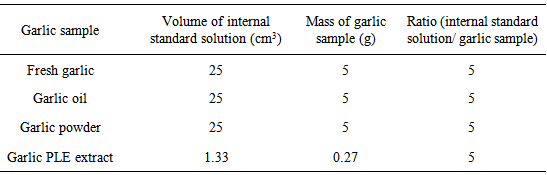
- In addition to the samples of the garlic PLE extract, samples of fresh garlic (Allium sativum L.), garlic oil (International Flavors & Fragrance Inc., Sao Paulo, Brazil) and garlic powder (Fuchs, Gewürze, Brazil) were analyzed. The garlic sample preparation was performed according to Eagling and Sterling [8]. For this, garlic samples were dissolved in the internal standard solution with magnetic stirring for 5 min. The ratio between the volume of the internal standard solution and the mass of the garlic sample was set to 5 (Table 1).
|
|
2.3.3. High-Performance Liquid Chromatography Analysis
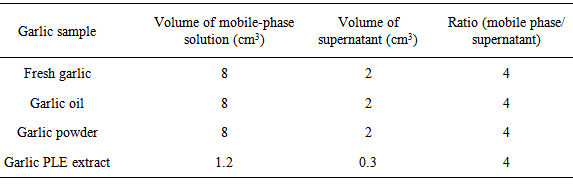
- For the high-performance liquid chromatography (HPLC) analyses, the mixtures of the mobile-phase solution and supernatant were filtered through 0.25-µM nylon syringe filters (VWR-International, Darmstadt, Germany) and were placed directly into HPLC vials. The chromatographic separation was performed using a Waters Alliance separation module (model 2695D, Milford, MA, USA) equipped with a diode array detector (2998). The separation was performed using a Poroshell C18 column (100 × 4.6 mm id, 2.5 µm, Agilent Technologies, Sunnyvale, CA, USA) at 323 K using a flow rate of 0.4 cm3.min-1. The initial mobile-phase consisted of water containing 0.1% acetic acid (solvent A) and methanol (solvent B). The mobile-phase composition of 50% A and 50% B was maintained for 12 min. The concentration of solvent A was decreased from 50% to 10% in 1 min, and this concentration was maintained for 5 min. Then, the concentration of solvent A was increased to its initial value (50% solvent A) in 2 min. The injection volume was 10 µL. UV detection was performed at 254 nm.
2.3.4. Identification of Allicin in the Garlic Samples
- The allicin in the garlic samples was identified using the UV spectrum of pure allicin that was found in the literature [20] and by the order of allicin elution with respect to that of the internal standard [21].
2.3.5. Allicin Quantification
- Allicin quantification in garlic extract samples was performed using ethyl p-hydroxybenzoate as the internal standard. The allicin concentration in the garlic samples was calculated using Equation 2 [21].
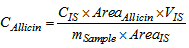 | (2) |
3. Results and Discussion
3.1. Preparation of the Plant Material
- The agglomeration phenomenon, also called the caking phenomenon, consists of small particles aggregating to form dense lumps. This phenomenon occurs when the processing temperature rises above the glass-transition temperature (Tg) of a material [22]. Studies have related this phenomenon to the moisture content of a solid matrix. The caking phenomenon is commonly observed in vegetal matrixes that have high water content [23]. A study of a large variety of Brazilian garlics determined that the water content of fresh garlic ranges from 65 to 70% [24]. Additionally, small particles and high temperatures favor the occurrence of the caking phenomena [25]. Allicin is a very labile compound that readily decays, particularly at high temperatures, and drying allicin-containing materials (to reduce the water content) at a high temperature or even at a low temperature, such as when freeze-drying, can result in a significant reduction in the level of allicin [26]. Thus, in this study, to reduce the extent of agglomeration without promoting allicin degradation, fresh garlic cloves instead of dried garlic were used. Additionally, to prevent caking, the fresh garlic was cut into small cubes using a knife instead of crushing it until it formed sticky lumps.
3.2. Pressurized Liquid Extraction
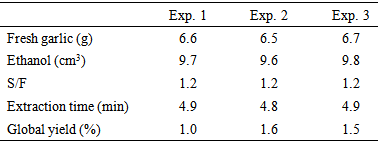
- PLE was performed at 313 K and 6 MPa. A low temperature was used to avoid allicin degradation. A pressure of 6 MPa was used because it is the maximal pressure at which the unit can operate in the dynamic mode.The selection of an extraction solvent was based on the solubility and stability of allicin. Fujisawa et al. [12] investigated extracting allicin from garlic using several different solvents at low pressure. The authors concluded that ethanolic solutions, of various concentrations (20–100%) produced extracts with higher allicin yields than those obtained using water, n-hexane or vegetable oil. Moreover, they concluded that allicin is more stable in ethanolic solutions than in water and that it is very unstable in vegetable oil. Ilić et al. [9] reported that allicin and other thiosulfinates could be transformed into more stable compounds when they were in non-polar organic solvents, such as n-hexane and oil.Therefore, due to the instability of allicin in non-polar organic solvents and the high yield of allicin obtained using ethanolic solutions, ethanol was used as the solvent in this study. Because the garlic samples were wet, bed extraction was performed within the extraction vessel after placing the sample particles without compacting them.The global yield (X0,S/F) of PLE extraction for fresh garlic was 1.3 ± 0.3% on a wet basis. More information about the extraction assays is shown in Table 3.
|
3.3. Identification of Allicin in the Garlic Samples
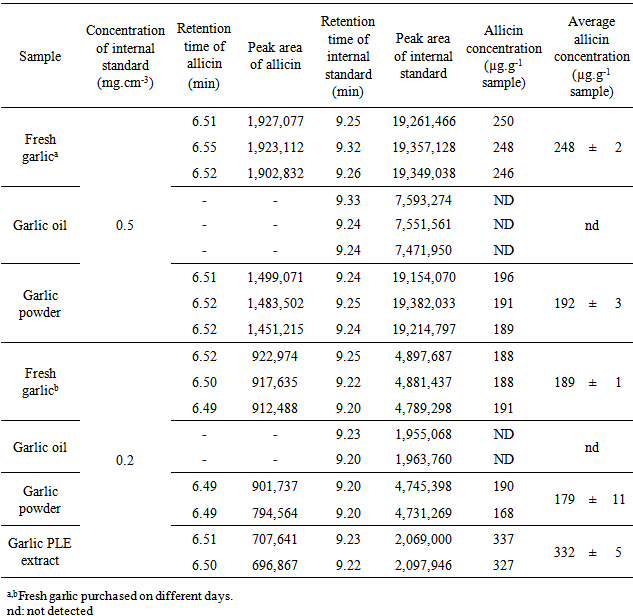
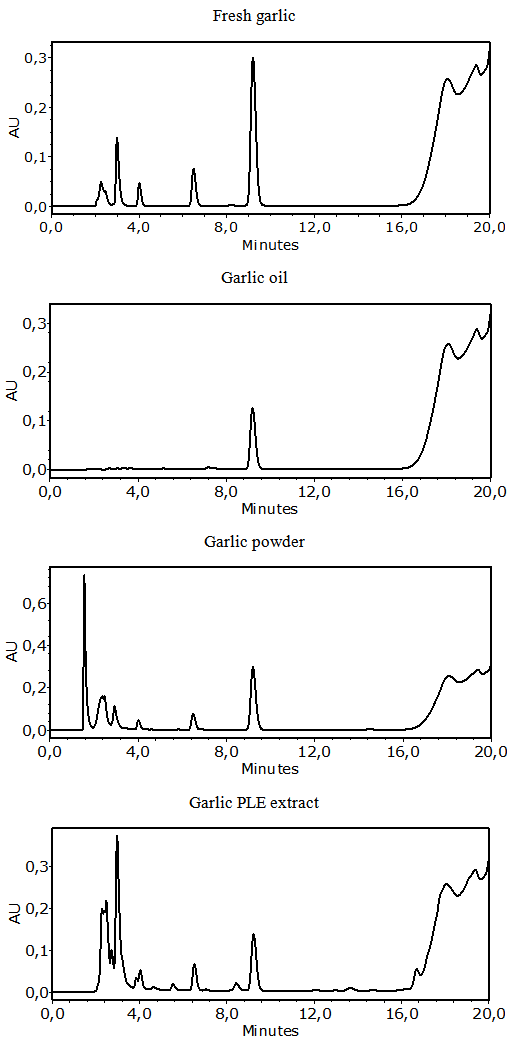
- The main peaks in the UV chromatograms of all of the garlic samples (Figure 3) were analyzed. The UV spectrum of allicin was identified and it is shown in Figure 4. The typical shoulder of the allicin peak at 240 nm was observed [27]. The retention times (tR) of allicin and ethyl p-hydroxybenzoate (used as the internal standard) were 6.5 and 9.2 min, respectively. The order of elution, with allicin eluted before the internal standard, was as expected [21].
 | Figure 3. Chromatograms of the garlic samples obtained using 0.2 mg.cm-3 of the internal standard; the allicin tR = 6.5 min and the internal standard tR = 9.2 min |
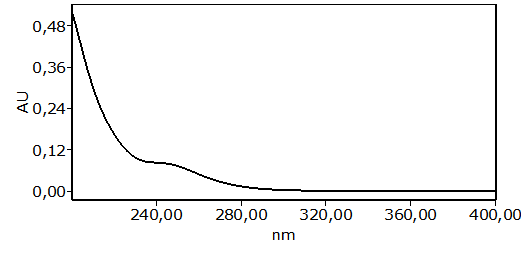 | Figure 4. UV spectrum of allicin that was identified in the garlic samples |
|
- The garlic PLE extract obtained at 313 K and 6 MPa, using an S/F of 1.2, had the highest allicin concentration: 332 ± 5 µg of allicin.g-1 of sample, followed by the fresh garlic and garlic powder. Allicin was not detected in garlic oil samples, most likely because its instability in non-polar solvents as explained. There are two reasons for the high allicin concentration in the sample of garlic PLE extract. First, allicin is more soluble in ethanol than in water (the other garlic samples were obtained using water). Second, allicin is stable in ethanol because ethanol contains a hydroxyl group that stabilizes the structure of the allicin molecule.Another environmentally friendly extraction method is supercritical fluid extraction (SFE). del Valle et al. [25] studied the extraction of allicin from garlic by SFE using carbon dioxide as the solvent. The optimal extraction conditions was determined to be 323 K, 30 MPa and S/F of 55, and extracts with allicin concentration of 75 µg of allicin.g-1 of extract were obtained. The low allicinconcentration in the SFE extracts could be due to the instability of allicin in garlic oil, which forms the SFE extract. Thus, by comparing of PLE and SFE techniques for obtaining extracts of garlic, it can be concluded that the PLE provides extracts with a higher concentration of allicin (332 ± 5 µg of allicin.g-1 of extract and 75 µg of allicin.g-1 of extract, respectively) and results in a lower consumption of solvent (S/F of 1.2 and 55, respectively) compared with the SFE.Several other advantages of extracting garlic using the PLE technique are the co-extraction of other polar compounds, such as phenolics, which aresimilar to allicin, have biological activities and the obtaining of extracts with a low content of garlic oil (which is responsible for the garlic odor) due to the low solubility of oil in ethanol, which have applications in the food, cosmetic and pharmaceutical industries.
4. Conclusions
- The global extraction yield of garlic PLE at 313 K, 6 MPa and S/F of 1.2 was 1.3 ± 0.3% on a wet basis. The garlic PLE extract had a higher allicin concentration (332 ± 5 µg of allicin.g-1 of extract) than fresh garlic and garlic powder samples. PLE is therefore an effective method for obtainingallicin-rich extracts.
ACKNOWLEDGEMENTS
- Angela M. Farías-Campomanes is grateful to CAPES (5817-11-0) for the Ph.D. assistantships. M. Angela A. Meireles is thankful to CNPq for a productivity grant (301301/2010-7). Claudia N. Horita and Marise A. R. Pollonio thank FAPESP (2011/51721-4). The authors acknowledge the financial support from CAPES, CNPq and FAPESP. We also would like to thank J. Felipe Osorio-Tobón for assistance with the HPLC analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML