-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2014; 4(5): 242-246
doi:10.5923/j.fph.20140405.05
Mineral Availability and Dietary Fiber Characteristics of Moringa oleifera
Aida C. Mallillin, Trinidad P. Trinidad, Rosario S. Sagum, Marco P. de Leon, Mellissa P. Borlagdan, Amster Fei P. Baquiran, James S. Alcantara, Theressa F. Aviles
Food and Nutrition Research Institute, Department of Science and Technology, Metro Manila, Philippines
Correspondence to: Trinidad P. Trinidad, Food and Nutrition Research Institute, Department of Science and Technology, Metro Manila, Philippines.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Background:There is an increasing awareness on the potential of Moringa Oleifera (Malunggay) as a high-value agricultural crop because of its high dietary fiber and nutrient contents. Objective: The study determined mineral availability and dietary fiber characteristics of Moringa Oleifera. Materials and Methods: Malunggay leaves and fruit from Metro Manila and two suppliers were analyzed. Proximate composition, minerals, phytic/tannic acid, total/soluble/insoluble fiber were analysed and subjected for mineral availability and dietary fiber fermentation in vitro. Results: All samples were good sources of protein (46.4 g/100g), ash (11.5 g/100 g) and beta-carotene (45172 µg/100g). Mineral content of malunggay leaves powder were: calcium (1.3±0.1-2.0± 0.0 g/100g) > iron (5.7±0.2-7.8± 0.0mg/100g) > zinc (3.4±0.1-4.8±0.0 mg/100g; P<0.05). The fruit powder contained 4.1±0.1 mg iron, 3.2±0.0 mg zinc and 0.4±0.0 g calcium per 100 g. Phytic acid (368±06 -576±72 mg/100 g sample) and tannic acid (1280±24-1956±09 mg/100 g) from leaves and fruits were high. All products were good sources of total (24.3±0.2-39.9±0.2 g/100g), insoluble (19.6±0.2-34.9±0.2 g/100g) and soluble (3.1±0.2-5.0±0.2 g/100g) dietary fiber. Zinc availability (59.5±0.1–93.8±0.1%) from malunggay leaves was significantly greater than that of calcium (25.0±0.1-53.8± 0.2%) and iron (7.0±0.1-19.5±0.1%; (P<0.05). Similar trend was observed from malunggay fruit. Dietary fiber fermentation of malunggay leaves produced short chain fatty acids, propionate (161.8±5.0-210.7±13.1 mg/g) > acetate (1.4±0.1 - 21.7±0.1 mg/g) > butyrate (3.0±0.1-7.7±2.3 mg/g; P<0.05). Conclusions: Malunggay leaves and fruits are good sources of iron, zinc and calcium and moderate to highly available for absorption regardless of the high content of phytic and tannic acid. Malunggay leaves are excellent sources of dietary fiber, fermentable and produce short chain fatty acids with greater amounts of propionate shown to inhibit cholesterol synthesis.
Keywords: Moringa oleifera, Dietary fiber, Mineral, Mineral availability
Cite this paper: Aida C. Mallillin, Trinidad P. Trinidad, Rosario S. Sagum, Marco P. de Leon, Mellissa P. Borlagdan, Amster Fei P. Baquiran, James S. Alcantara, Theressa F. Aviles, Mineral Availability and Dietary Fiber Characteristics of Moringa oleifera, Food and Public Health, Vol. 4 No. 5, 2014, pp. 242-246. doi: 10.5923/j.fph.20140405.05.
1. Introduction
- Malunggay (Moringa oleifera) is the most widely cultivated species of a monogeneric family, the Moringaceae, that is native to the sub-Himalayan tracts of India, Pakistan, Bangladesh and Afghanistan. This rapidly-growing tree (also known as the horseradish tree, drumstick tree, benzolive tree, kelor, marango, mlonge, moonga, mulangay, nébéday, saijhan, sajna or Ben oil tree), was utilized by the ancient Romans, Greeks and Egyptians; it is now widely cultivated and has become naturalized in many locations in the tropics. It is a perennial softwood tree with timber of low quality, but which for centuries has been advocated for traditional medicinal and industrial uses. It is already an important crop in India, Ethiopia, the Philippines and the Sudan, and is being grown in West, East and South Africa, tropical Asia, Latin America, the Caribbean, Florida and the Pacific Islands. All parts of the Moringa tree are edible and have long been consumed by humans [1].Malungay is widely cultivated in the Philippines. It is a promising food source in the country because the tree is in full leaf at the end of the dry season when other foods are made typically scarce. Almost all year round, malunggay leaves are present and readily available for consumption.There is an increased awareness on the potential of malunggay as a high-value agricultural crop because it is widely known for its nutritional values and health claims. While much of the information appears to be justified, it is critical to separate rigorous scientific evidences from anecdotes and testimonials. The Philippine government has recently promoted a biotechnology program that encourages the planting and commercialization of malunggay to address iron and other micronutrient deficiencies. Hence, there is a need to assess the availability of minerals from malunggay for absorption in the body. On the other hand, interest in dietary fiber is growing due to the multiple benefits this dietary components has on health. The study determined mineral availability and dietary fiber characteristics of Moringa Oleifera. This study may contribute in the solution of the double burden malnutrition existing in developing countries like the Philippines.
2. Materials and Methods
- Sampling and Preparation of SamplesMalunggay leaves and fruit are available the whole year round and can be harvested anytime. The harvest time of the Malunggay samples used in this study was not known but the samples were fresh at the time of purchase. The samples were obtained from different markets in four parts (north, south, east and west) of Metro Manila. The purchased samples were stored in the refrigerator for pooling and sample preparation.Malunggay leaves were picked one by one and the stems and inedible parts were discarded. The samples were weighed, freeze-dried and pulverized.Malunggay fruit were dehusked and the meats including the seeds were pooled. Only the edible parts were taken from the malunggay fruit. The pooled malunggay fruit was freeze-dried and pulverized.Samples from two suppliers of malunggay leaves powder were also used in the study. The sources of Malunggay from the suppliers came from Southern Tagalog and Northern part of Philippines.Samples were analyzed immediately after freeze-dried at the Nutrient Availability Unit Laboratory, Food and Nutrition Research Institute-Department of Science and Technology, Philippines.Analytical Methods:Freeze-dried samples were analyzed for proximate composition, iron, zinc and calcium, total, soluble and insoluble dietary fiber using AOAC methods [2-4] as well as phytic acid [5] and tannic acid [6].In vitro mineral availability [7]Ten grams of the freeze-dried malunggay leaves samples and five grams of freeze-dried fruit samples were separately weighed in 250-mL Erlenmeyer flask. 100-g sample solutions were prepared by the addition of 90mL of double deionized water to malunggay leaves and 95mL to fruit samples. The samples were homogenized and the pH was adjusted to 2.0 using 6N HCl. Pepsin-HCl solution (3.2mL) was added and the resulting solution was incubated for three hours at 37℃ in a water bath shaker (100rpm). From the digested sample solution, twenty grams was aliquoted and added 5mL of pancreatin-bile solution. The pH was adjusted to 7.5 using 0.5M KOH. Four times the volume of the KOH used was equivalent to the volume of 0.5MNaHCO3 in 100mL dialysis solution. Another 20g of pepsin-digested sample solution was weighed in a dialysis bag (Spectrum Lab) plus 5mL pancreatin-bile solution. The dialysis bag was immersed in the bicarbonate solution. The mixture was incubated for a total of 12 hrs at the same condition as the pepsin digestion. The dialysates were collected every 3 hrs, replacing with 100mL of double deionized water. The dialysates were analyzed for mineral content using AAS.In vitro fermentation of dietary fiber [8]Freeze dried malunggay leaves and fruit will be fermented in vitro using human fecal inoculum. A 0.5 g of the test food will be weighed in a serum bottle. Forty (40) mL of fermentation media (mix 2L deionized water, 1L 0.5M sodium bicarbonate buffer solution, 1L macromineral solution, 5 mL of 0.1% resazurin), and 2 mL reducing solution (a mixture of 1.25 g cysteine-HCl+50 pellets of potassium hydroxide in 100 mL deionized water and 1.25 g sodium sulfide in 100 mL deionized water) will be added to the serum bottle and flushed with carbon dioxide until colorless. The bottles will be sealed with rubber stoppers and aluminum seal and stored at 4℃. Samples are sealed with rubber and aluminum cap prior to storage at 4℃.The next day the bottles will be placed in a water bath for 1-2 hours at 37℃. A 10-mL fecal inoculum (1:15 dilution of fresh feces from a human volunteer) will be added into each bottle and the mixture will be incubated for 24 hours at 37℃. The fermented digest will be filtered and read in a high pressure liquid chromatography (HPLC) to measure short chain fatty acids against a volatile acid standard mix (SUPELCO, Philadelphia, USA).Statistical AnalysisDifferences between samples were determined by repeated measures analysis of variance and Duncan’s Multiple Range Test using SPSS.
3. Results
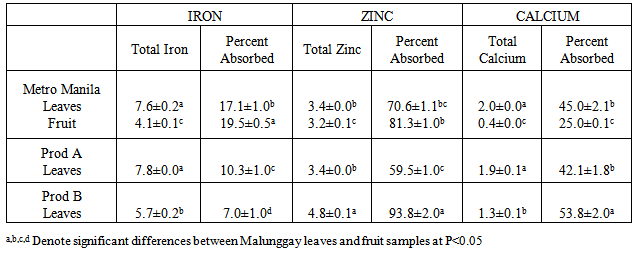
- Differences in nutrient contents of the three samples of malunggay leaves were observed and may be attributed to the soil and location where malunggay was grown (Table 1). Malunggay leaves were found to be good sources of protein, ash and β-carotene. These results are comparable to another study (1).
|
|
|
|
|
4. Discussion
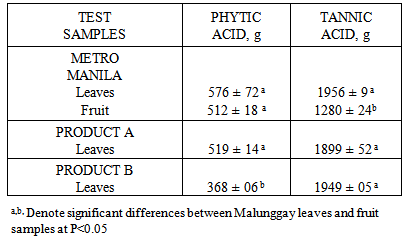
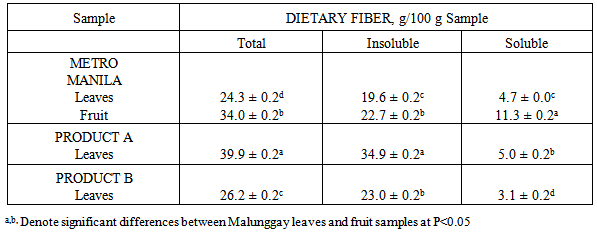
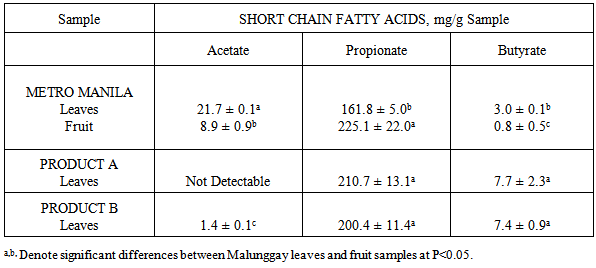
- Results from this study showed that the mineral content of malunggay leaves differed from different sources similar to another study [9]. Factors to consider are the site and the mineral content of the soil where malunggay is planted. In general, the total content of a mineral in soil is a very broad guide to its plant availability. The plant availability of minerals depends on the soil pH. For example, zinc are plant available over a soil pH range of 5-7 but iron have greater availability below pH 6 while calcium is more available above pH 6.5 [10]. The mineral content of the soil where the test samples are planted was not determined in this study.The availability of calcium, zinc and iron from malunggay leaves and fruit for absorption in the body may not be affected by the high phytic acid, tannic acid it contain. The vitamin C content of malunggay leaves of 231 mg/100 g sample [11] may be considered high enough to overcome the actions of inhibitors of calcium, zinc and iron absorption e.g. phytic and tannic acid. Vitamin C (ascorbic acid-AA) has been shown to enhance iron and zinc absorption [12]. Vitamin C is the most efficient enhancer of non-heme iron absorption and the presence of ascorbic acid is more pronounced when a food/meal is at a molar ratio of AA to iron of 2:1 in the presence of low to medium levels of iron inhibitors [13, 14]. However, the ratio of AA to iron in this study is 10:1 while AA to zinc ratio is 19:1. The higher ratio of AA to iron as well as to zinc in this study may have overcome the inhibitory effect of phytic and tannic acid on iron and zinc availability. Because of the high calcium content as well as ascorbic acid content of malunggay observed in this study, the inhibitors of calcium availability was not able to do its actions. Therefore the lesser availability of iron from malunggay may be due to mineral-mineral interaction. The high amounts of calcium may have caused the low iron availability observed from this study.The differences in the dietary fiber content of the different malunggay leaves may be due to their particle size. Particle size was found to affect the dietary fiber content of food. The coarse food particles have higher dietary fiber content than that of fine food particles [15]. However, measurement of particle size e.g. mesh size, was not done in this study.The growing interest on the role of dietary fiber has been attributed to its nutritional and health benefits [16]. In this study, it was found that both malunggay leaves and fruit are excellent sources of dietary fiber. The dietary fiber was fermentable producing the short chain fatty acids (SCFA) with propionate as the highest SCFA produced. This has some important implications in propionate’s role to inhibit the limiting enzyme HMG coenzyme-reductase for cholesterol synthesis [17]. Dietary fiber has also a significant role in slowing down the release of glucose after digestion since we do not have an enzyme to degrade it in the small intestine, thus is metabolized by the microorganisms in the colon e.g. lactobacillus and bifidobacteria. This action of dietary fiber may be recommended in the proper control and management of obesity and type II diabetes mellitus [18].Mineral availability in the presence of dietary fiber may not result in the reduction of available minerals for absorption if the dietary fiber is fermentable [7]. Minerals can bind with dietary fiber in the small intestinal conditions but if the dietary fiber is fermentable in the colon can be released for absorption in the colon [7]. This may be true for malunggay’s high fiber-high mineral interaction observed in this study. In conclusion, dried malunggay leaves and fruit are good sources of iron, zinc and calcium considered to be moderate to highly available for absorption in the body. Malunggay leaves and fruits are excellent sources of dietary fiber, fermentable and produced greater amounts of propionate suggesting protective effect for cholesterol synthesis. Human mineral absorption and efficacy studies are recommended to validate the above results.
ACKNOWLEDGEMENTS
- The authors would like to thank the following FNRI staff for their technical assistance: Ms. Kristine Bernadette B. Cid, Mark Ryan Q. Ibardaloza, and Zoilo B. Villanueva. This study is funded by the Department of Science and Technology (DOST) through the Philippine Council for Health Research and Development-DOST.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML