-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2014; 4(5): 209-213
doi:10.5923/j.fph.20140405.01
Determination of total Aflatoxins and Carbamate Pesticide Residues in Some Bee Honey Samples Using QuEChERS Method and High Performance Liquid Chromatography
Asmaa A. Eissa1, Ayman S. M. Hassan2, Tarek A. Abd El Rahman2
1Plant Protect. Res. Inst. Giemeza, Agric. Res. St. Agric. Res. Center
2Central Agricultural Pesticides Laboratory, Agricultural Research Center ARC, Dokki, Giza, Egypt
Correspondence to: Tarek A. Abd El Rahman, Central Agricultural Pesticides Laboratory, Agricultural Research Center ARC, Dokki, Giza, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
This study presents the evaluation of aflatoxins (B1, B2, G1, and G2) and carbamate pesticide contamination in 44 samples of bee honey were locally produced, in Egypt and other countries (Kuwait, Saudi Arabia, China, Turkey, Ukraine, Libya, Ethiopia, Italy and USA) during 2012–2013 using Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) method. The presence of total aflatoxins and carbamate pesticides residues were determined by high-performance liquid chromatography (HPLC) with fluorescence and UV- diode array (DAD) detectors, respectively. The recovery results of total aflatoxins and carbamate pesticides were found to range from 88.25% to 92.9% and 78.49% to 98.11%, respectively. The results indicated that, all collected samples were free from any detectable residues of aflatoxins. On the other hand, promocarb, pirimicarb and aldicarb residues were found in few bee honey samples. All contaminated bee honey samples with carbamate pesticides were under maximum residue limit (MRL’s).
Keywords: Bee honey, Aflatoxins, Carbamate pesticides, QuEChERS, HPLC
Cite this paper: Asmaa A. Eissa, Ayman S. M. Hassan, Tarek A. Abd El Rahman, Determination of total Aflatoxins and Carbamate Pesticide Residues in Some Bee Honey Samples Using QuEChERS Method and High Performance Liquid Chromatography, Food and Public Health, Vol. 4 No. 5, 2014, pp. 209-213. doi: 10.5923/j.fph.20140405.01.
Article Outline
1. Introduction
- Honey is an interesting food that can be used as an ingredient or as a final product [1]. It is a highly-energetic natural carbohydrate produced when the nectar and sweet deposits from plants, gathered, modified and stored in the honey comb by honey bees [2]. The quality of honey is mainly determined by its sensorial, chemical, physical and microbiological characteristics. Honey physicochemical quality criteria are well specified by the EC Directive 2001/110 [3]. Bee products, such as honey, are widely consumed as food and medicine and their contamination may carry serious health hazards. Certain fungi that can grow on food such as dried fruits, nuts, and cereals, legumes and spices produces naturally-occurring toxins called Mycotoxins. The most commonly observed mycotoxins that found are aflatoxins (B1, B2, G1 & G2) and ochratoxin-A. Aflatoxins directly damages DNA and have been shown to cancer contribution to food contamination, including mycotoxins. Aflatoxins could cause liver damage in the laboratory [4], besides the economic loss due to be more hazard to human health. They are carcinogenic, toxigenic, teratogenic and mutagenic [5]. There are few information concerning mycological contamination and simultaneous co-occurrence of Asp. flavus or Asp. parasiti cus and aflatoxins detection in honey. Studied fungal growth and aflatoxin production on apiarian substrates unprocessed honey, pollen, broad comb, whole larvae and whole bees, and verified that fungi grew on and produced aflatoxins in low levels in all substrates except the unprocessed honey [6]. Another similar study carried out by inoculated unprocessed honey with toxigenic strains of Asp. flavus NRRL 5862 and Asp. parasiticus NRRL 2999, the fungal growth was observed, but none of the cultures produced detectable levels of Aflatoxins [7]. Agricultural contamination with pesticides and antibiotics is a challenging problem that needs to be fully addressed. In recent years, analysis of pesticide residues in food becomes an essential requirement for consumers, producers, and food quality control authorities [8]. Honey and other bee products are polluted by pesticides, heavy metals, bacteria and radioactive materials. Pesticide residues cause genetic mutations and cellular degradation and the presence of antibiotics might increase resistant human or animal’s pathogens [9]. Many cases of infant botulisms have been attributed to contaminated honey. Honey may be very toxic when produced from certain plants. Ingestion of honey without knowing its source and safety might be problematic [9]. Honey should be labeled to explore its origin, composition, and clear statement that it is free from contaminants. Honey that is not subjected for analysis and sterilization should not be used in infants, and should not be applied to wounds or used for medicinal purposes [9]. However, the results of using heavy application of pesticides may contribute to pesticide residues above their respective maximum residue limit (MRL) which may pose health hazards to consumers [9].Carbamate insecticides have a similar mode of action with organophosphates but their insecticidal activity is more selective and depends to a certain extent on the insect species. Some fungicides and herbicides belong to this family. These substances are highly volatile in the environment and in some cases they were detected in beehive products [10].Various studies were monitoring residues of carbamate pesticides on honey and other bee products [10, 11]. The concentration of carbamate residues detected in pollen ranged from 0.126 mg kg-1 to 0.265 mg kg-1 for the active ingredient carbaryl, while the maximum concentration of carbofuran was 0.14 mg kg-1 [10]. Concentration of carbaryl, carbofuran, pirimicarb and methiocarb residues, in most cases is considered low and does not exceed 0.071 mg kg-1. In only one Spanish honey the concentration of carbofuran was 0.645 mg kg-1 [11].The present work was carried out to study monitoring of total aflatoxins and carbamate pesticides residues in bee honey samples collected during 2012–2013 and comparing the obtained results with the published MRL values.
2. Material and Methods
2.1. Sampling
- A total of 44 samples of bee honey were collected at local markets from different countries (Egypt (8 samples) and (4 samples) for each country (Kuwait, Saudi Arabia, China, Turkey, Ukraine, Libya, Ethiopia, Italy and USA)) during 2012–2013. All samples collected were local produced of each country studied. Samples were obtained through the Arab Bees Union and Bee Breeders Association Gharbia Governorate in Arab Republic of Egypt. Not less than 500 grams of honey were taken for each sample in jar labeled by name of market and country then transferred to the Lab. Samples were divided into five portions consisting of 100 g each were taken, four samples for extraction and one was kept in deep freezer at -20°C. Extraction was carried out as soon as possible.
2.2. Chemicals and Materials
- Standards of aflatoxins B1, B2, G1 and G2 (Fig. 1) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Standard stock solutions were prepared in methanol. Working solutions were prepared by diluting the stock standards with mobile phase to the final mass concentration of 0.5 μg of each aflatoxin B1and B2/ml and 1.0 μg of each aflatoxin G1 and G2 /ml. The QuEChERS extraction comprised sodium chloride, trisodium citrate 5.5-hydrate, and anhydrous magnesium sulfate, anhydrous sodium acetate, and methanol, acetonitrile and 99.5 % acetic acid (Sigma–Aldrich, Germany).
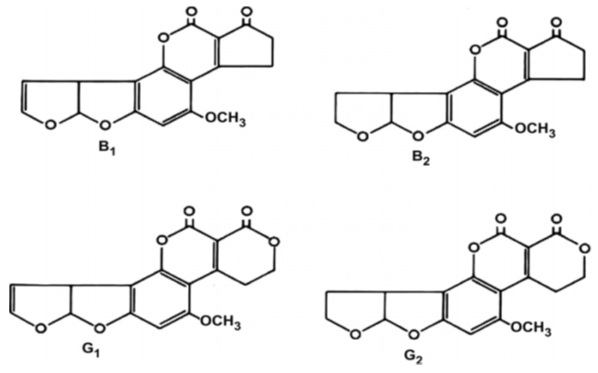 | Figure 1. Chemical structures of aflatoxins B1, B2, G1 and G2 |
2.2.1. Carbamate Pesticides Used
- Seventeen pure standards of the most important carbamate pesticides were used for identification and quantification, the detected residues included: Kresoxim-ethyl, Furathiocarb, Mexacrbate, Fenoxycarb, Vernolate, Aldicarb, Thiodicarb, Methomyl, Propoxur, Bendiocarb, Carbofuran, Ethofumesate, Chlorufum, Methiocarb, Pirimicarb, Carbaryl and promocarb. The average recoveries percentage of aflatoxin and pesticides for 3 spiked levels (0.05, 0.01, and 0.001mg/kg) in bee honey samples were conducted (Table 1, 2).
|
2.3. Analysis of total Aflatoxins and Carbamate Pesticides
- The samples were comminuted (10 g) of each was then placed into 50 mL polyethylene tube. Samples were extracted and cleaned up immediately after sampling using QuEChERS methodology [12,13]. Fifteen mL of acetonitrile and 5 mL of deionized water were added into each tube. The samples were well shaken using a vortex mixer at maximum speed. Afterwards, 6 g of anhydrous magnesium sulfate and 1.5 g of sodium chloride were added, then extract by shaking vigorously on vortex for 5 min and centrifuged for 10 min at 4,000 rpm. An aliquot of 4 mL was transferred from the supernatant to a new clean 15 mL centrifuge tube containing 100 mg PSA and 600 mg anhydrous magnesium sulfate. The samples were again vortexed for 3 min and then centrifuged for 10 min at 4,000 rpm.
2.3.1. Derivatization
- Aflatoxin were derivate by adding 50 µL of TFA and 200 µL hexane to the residue and vortex-mix vigorously for 30 sec. After 5 min, 1.95 mL of acetonitrile: water (1:9) was add and vortex-mix vigorously for 30 sec. The sample centrifuged for 3 min at 4000 rpm. The lower aqueous layer used for HPLC determination.
2.3.2. HPLC Analysis
- The HPLC analyses were carried out with Agilent 1100 system, consisting of a degasser, binary pump, auto sampler, column oven, UV- DAD and a fluorescence detector. The chromatographic separation was performed with the Zorbax EclipsePlus C18 (3.5μm, 3.6 mmx150 mm) chromatographic column. In the case of aflatoxin, the mobile phase was deionized water-methanol-acetonitrile (65:23:12), at a flow rate of 1 mL/min. aflatoxin were detected by fluorescence detector at the excitation and emission wavelengths of 360 nm and 440 nm, respectively. The injection volume was 25 µL [14].While for carbamate residues, the mobile phase was deionized water containing 0.1% formic acid (mobile phase component A) and acetonitrile (component B) were employed for the gradient program, which started with 20% B for 3 min and was linearly increased to 100% B in 27 min (held for 3 min). The column was then re-equilibrated for 12 min back to 20% B. Thus, the total run time took 45 min. The flow rate was constant at 0.6 mL/min, and injection volume was 10 μL [8].
2.4. Statistical Analyses
- Data analyses were performed using Statistical Package for the Social Sciences (SPSS version 12.0). Values were expressed as percentage, range, minimum, maximum and mean appropriate test statistics (ANOVA) were done to determine aflatoxin and carbamate pesticides content in bee honey samples.
3. Results and Discussion
3.1. Monitored Total Aflatoxin Residues in Bee Honey
- In this study 44 samples of bee honey were analyzed for total aflatoxin. The result of the samples collected from Egypt and other countries (Kuwait, Saudi Arabia, China, Turkey, Ukraine, Libya, Ethiopia, Italy and USA) during 2012–2013. All samples were free from any detectable aflatoxin (B1, B2, G1 and G2). Maximum residue limit (MRL) of B1 and total aflatoxin were 5 µg/kg and 10 µg/kg, respectively.Our results agreed with [15], their study holds on 80 samples of honey, randomly collected from retail markets. They were concerned with the contamination with Bcilaceae spores (Clostridium perfringens, Bacillus cereus), fungi and aflatoxins. The microflora was determined using conventional microbiological methods and the aflatoxins were detected by HPLC. Spores of Clostridium perfringens were not detected in any sample. None of samples revealed to be contaminated with aflatoxins. However, we disagree [16] where, they evaluated aflatoxins (B1, B2, G1, G2) and heavy metals (contamination in branded and unbranded honey). They reported that, the contamination level of aflatoxins (B1, B2, G1 and G2) was also evaluated in both types of honey. Minimum level of aflatoxins were detected in branded and unbranded honey samples (B1and B2 with 2.14, 1.25 µg/kg) and maximum concentration were (2.33, 2.15 µg /kg), respectively.
3.2. Monitored Carbamate Pesticide Residues in Bee Honey Samples
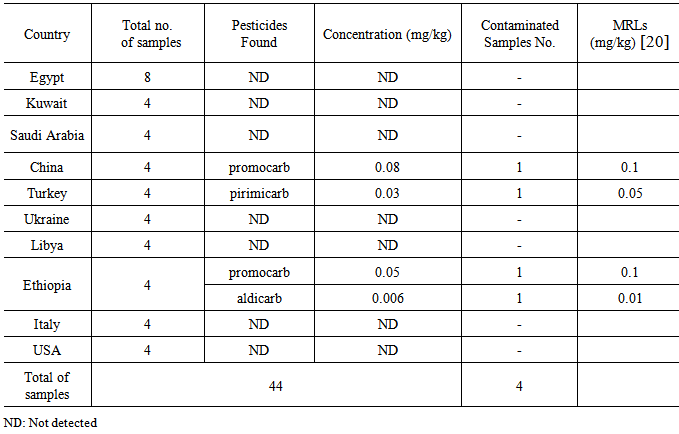
- The carbamate pesticide residues found in bee honey samples results are shown in Table 3. It is shown that, a total of 44 bee honey samples are analyzed. Residues of 3 active substances were found in 4 samples of bee honey. The most frequent residues identified were promocarb (in two samples), pirimicarb and aldicarb in one sample. Total contamination with pesticide residues was found in four samples. The highest contamination of pesticides was found in Ethiopia (2 samples), followed by Turkey and China (one sample). All samples of bee honey were under MRL’s of carbamates. All collected bee honey samples collected from countries (Egypt, Kuwait, Ukraine, Saudi Arabia, Italy, USA and Libya) were free from any detectable residues of pesticides. These findings are in agreement with those obtained by others [11, 17, 18 and 19]. In Spain, residues of thymol were found in honey collected from the beehives, and ranged from 0.75 to 8.20 μg/kg for Apilife Var [11]. Fifty samples of honey collected from local markets of Portugal and Spain during year 2002 were analyzed for various pesticides which included 42 organochlorine, carbamate, and organophosphorus [17]. It was found that Portuguese honeys were more contaminated than the Spanish [18]. In France, a field survey was initiated in French apiaries in order to monitor the health of honey bee colonies. Beeswax samples were collected once a year over 2 years from a total of 125 honey bee colonies. Residues of 14 of the searched compounds (16 insecticides and acaricides and two fungicides) were found in samples; taufluvalinate, coumaphos, and endosulfan residues were the most frequently occurring residues. Beeswax contamination was the result of both in-hive acaricides treatments and environmental pollution [19].
|
4. Conclusions
- Although some of the samples showed promocarb, pirimicarb and aldicarb residues were found in few bee honey samples, all collected samples were free from any detectable residues of aflatoxins .All contaminated bee honey samples were under MRL’s.
ACKNOWLEDGMENTS
- The authors are grateful to the Central Agricultural Pesticides Laboratory in Dokki, Giza, Egypt and Arab Bees Union and Bee Breeders Association Gharbia Governorate in Arab Republic of Egypt for technical support, academic advice, and instruction materials.
References
| [1] | Snowdon J.A. and Cliver D.O. (1996). Microorganisms in honey. Review article. Int. J. Food Microbial. 31: 1-26. |
| [2] | Laila A. Nasser (2004). Isolation and characterization of fungi contaminating packaged honey commonly consumed in Saudi Arabia. Ass. Univ. Bull. Environ. Res. 7(1): 1-7. |
| [3] | EU, 2001.Council Directive 2001/110 relating to honey. Official Journal of the European Communities. |
| [4] | Matthews, W. (2005). Survey Report. Food standard agency, Chemical safety division. London, UK. P. 2. |
| [5] | Hsieh, DPH. (1986). The role of aflatoxins in human cancer, Elsevier Science Publishers, Amsterdam; The Netherlands, 17(3): 447-456. |
| [6] | Hilldrup, J. A. L., Eadie, T. and Llewellyn, G. C. (1977). Fungal growth and aflatoxin production on apiarian substrates. J.A.O.A.C. 60, 96-99. |
| [7] | Wellford, T. E., Eadie, T. and Llewellyn, G. C. (1978). Evaluation the inhibitory action of honey on fungal growth speculation in aflatoxin production. Z. Lebensm Unters Forsch. 28, 166, 280-283. |
| [8] | Ahmed, M.A.I., N.S. Khalil and T.A. Abd El Rahman. (2014). Carbamate pesticide residues analysis of potato tuber samples using high-performance liquid chromatography (HPLC). Journal of Environmental Chemistry and Ecotoxicology. 6(1): 1-5. |
| [9] | Noori Al-Waili, Khelod Salom, Ahmed Al-Ghamdi, and Mohammad Javed Ansari. (2012). Antibiotic, Pesticide, and Microbial Contaminants of Honey:Human Health Hazards. The ScientificWorld Journal. Volume Article ID 930849, 9 pages. |
| [10] | Chauzat, M.P.; Faucon, J.P.; Martel, A.C.; Lachaize, J.; Cougoule, N. & Aubert, M. (2006). A survey of pesticide residues in pollen loads collected by honey bees in France. J. Econ. Entomol. 99 (2):253-262. |
| [11] | Blasco, C.; Fernandez, M.; Pena, A.; Lino, C.; Silveira, MAI.; Font G. & Pico Y. (2003). Assessment of pesticide resdues in honey samples from Portugal and Spain. J. Agric. Food Chem. 51:8132-8138. |
| [12] | Anastassiades. M, Lehotay, S.J, Stajnbaher. D (2002). Quick, easy, cheap, effective, rugged, and safe (QuEChERS) approach for the determination of pesticide residues. In 18th Annual waste is testing and quality symposium proceeding. Arlington, VA: 231–241. |
| [13] | Vojislava P. Bursić, Gorica Lj. Vuković, Igor M. Jajić, Sanja D. Lazić, Magdalena H. Cara, Radmilo R. Čolović, Đuro M. Vukmirović (2013) .Analysis of aflatoxins B1 and G1 in maize by QUECHERS. Jour. Nat. Sci, Matica Srpska Novi Sad,124: 51—57. |
| [14] | Tarek. A. Abdel-Rahman, Rania. M. Abd ElHamid, Fayza. A. Sdeek and Aly A. Shalaby (2014) Monitoring of aflatoxin in medicinal plants marketed in Egypt. Egypt. J. of Appl. Sci., 29 (6): 154:161. |
| [15] | Hermínia Marina Martins, M. Lígia Martins, Fernando M. A. Bernardo (2003). Bacillaceae spores, fungi and aflatoxins determination in honey. REVISTA PORTUGUESA de CIÊNCIAS VETERINÁRIAS, RPCV 98 (546): 85-88. |
| [16] | Khaliqur Rahman, Imdadullah muhammadzai, Arshad Hussain, HalimurRahman, Javid Ali (2014). Contaminants Analysis of Different Branded and Unbranded Honey of khyber pukhtounkhwa Pakistan. Life Science Journal. 11(3s): 227-231. |
| [17] | Beyoǧlu. D and G. Z. Omurtag. (2007). Occurrence of naphthalene in honey consumed in Turkey as determined by high-pressure liquid chromatography. Journal of Food Protection. 70 (7): 1735–1738. |
| [18] | Adamczyk, S. R. Lázaro, C. Pérez-Arquillué, P. Conchello, and A. Herrera. (2005). Evaluation of residues of essential oil components in honey after different anti-varroa treatments. Journal of Agricultural and Food Chemistry. 53(26): 10085–10090. |
| [19] | Chauzat M. P. and J. P. Faucon. (2007). Pesticide residues in beeswax samples collected from honey bee colonies (Apis mellifera L.) in France. Pest Management Science. 63(11): 1100–1106. |
| [20] | http://ec.europa.eu/sanco_pesticides/public/index.cfm?event=commodity.resultat (2012). |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML