-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2014; 4(4): 204-208
doi:10.5923/j.fph.20140404.05
Toxicity Test of Alginate from Sargassum and Padina on the Liver of Mice
Wahyu Mushollaeni, Nonok Supartini, Endang Rusdiana
Agroindustrial Technology, Tribhuwana Tunggadewi University, Malang, East Java, 65144, Indonesia
Correspondence to: Wahyu Mushollaeni, Agroindustrial Technology, Tribhuwana Tunggadewi University, Malang, East Java, 65144, Indonesia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
This research aimed to study the toxicity of alginate Sargassum and Padina with animal (mice) testing. This research is an experimental approach to post-test only control group design. Wistar rats used as an object of alginate toxicity testing. The mice were grouped into 5, i.e. control group (no alginate diet), 2 groups treated by Sargassum 0,75% and 1%, 2 groups treated by Padina 0,75% and 1%, respectively. Based on the research, it was concluded that alginate from Sargassum and Padina not give toxic effects on Wistar mice and no significant effect on liver cell damage.
Keywords: Sargassum, Padina, Alginate, Mice, Toxicity
Cite this paper: Wahyu Mushollaeni, Nonok Supartini, Endang Rusdiana, Toxicity Test of Alginate from Sargassum and Padina on the Liver of Mice, Food and Public Health, Vol. 4 No. 4, 2014, pp. 204-208. doi: 10.5923/j.fph.20140404.05.
Article Outline
1. Introduction
- Alginate has an important role in the food industry. The use of alginate as a food additive in the food industry with regard to the nature of the rough, such as a thickener [16] [18] [26], so that the product is more stable [8] [10] [19], stabilize mixtures, dispersions and emulsions with regard to its nature as a gelling and viscosity increase [24].Although alginate has been known and used in various applications, especially in food purposes, but there is no toxicity studies that examine the effects of toxicity associated with the possible presence of harmful chemical residues present in the extract alginate applied to food products. Toxicity and health effects of using test animals that Wistar rats or mice (Rattus norvegicus). The toxicity of a substance can be determined by using the test animals are male or female white rat strain Whistar [2][3][30]. The liver is an organ that did the metabolism and detoxification of toxins from the entry of foreign material into the body [12].Dietary fiber particularly one which is soluble in water, is known to play a role in lowering plasma cholesterol levels [5]. Alginate has a high potential in lowering blood cholesterol by inhibiting cholesterol absorption in the intestine [39]. The effect of sodium alginate, performed with mice test [2][3]. An important part of the subchronic toxicity testing is liver and kidney histopathology [40].The liver is an important organ that detoxifies many substances functioning digestive tract digestive results [37]. The main function of the liver is to metabolize and detoxify toxins [12]. Clinical features and diagnosis of drug-induced hepatotoxicity according to the Common Toxicity Criteria, covering grades 0-4 from increased alkaline phosphatase, increased bilirubin, increased GGT, hepatomegaly, hypoalbuminemia, clinical signs of liver dysfunction, decreased portal venous flow or retrograde, increased SGOT, and increased SGPT. One test that is often performed to determine the liver function is testing serum transaminase SGPT and SGOT are. Both serum is a sensitive indicator of damage to the liver cells. The damage to the liver cells can be characterized by the levels of enzymes SGOT (Serum Glutamic Oxaloasetate transaminase) and SGPT (Serum Glutamic Piruvate transaminase) were increased. SGOT-SGPT are two transaminase enzymes produced by the liver cells [14]. An increase in The enzyme aspartate aminotransferase (AST) and alanine aminotransferase (ALT) indicate liver cell damage compared with other liver enzymes, because this enzyme increases both first and increases dramatically when compared with other enzymes in the event of damage to the liver cells [7]. This research aimed to study the toxicity of alginate Sargassum and Padina with animal (mice) testing. Toxicity testing on food safety aspects of alginates in this study, using male mice strains Wistar.
2. Materials and Methods
2.1. Materials
- The raw materials used are 2 types of alginat i.e. Sargassum and Padina, derived from the rocky coast of South Mountain area of Yogyakarta. The chemicals used include distilled water, CaCl2 1% and 74%, HCl 1, 3, 5 and 35%, 0.5 and 90% KOH, Na2CO3 2.25; 10 and 95%, NaOCl 10 and 12%, IPA 95%, HNO3.
2.2. Population and Sample
- This study is divided into three stages, i.e: Extraction of alginates from brown seaweed Sargassum and Padina, Treatment period to wistar alloxan diabetic mice for 38 days, and Termination stage, blood sampling and blood test for SGPT and SGOT levels at the end of treatment.The population were mice (wistar specification). Samples were obtained in consecutive random sampling, with (a) the inclusion criteria of male Wistar mice, aged 3 months, weight 140-155 grams, healthy conditions (active and not disabled), and (b) exclusion criteria that mice experiencing pain, mice decreased weight (less than 140 grams), the mice died within the study period.Alginate to be tested, applied in the form of mackerel fish balls, which will be administered orally every day for 90 days. The concentration of alginate in mackerel fish ball is 0.75% (1) and 1% (2) for each of the 2 types of alginate. Control treatment (K) treated by mackerel fish balls (B) but without alginates.
2.3. Data Analysis
- Data analysis performed using specific program analysis for windows 16:00. Hypothesis testing using parametric One Way test (p <0.05).
2.4. Methods
- Alginate is applied as stabilizer in the form of a mice feeding mackerel fish balls. Parameter analysis in this study include SGPT and SGOT blood levels and body weight. Fish balls containing alginate administered orally every day for 90 days. The concentration of alginate in the mackerel fish ball is 0.75% (1) and 1% (2) for each of the two types of the alginate Sargassum (S) and Padina (P). Control treatment (K) is the mackerel fish balls without alginates. In this study using 25 mice and divided into 5 mice in each treatment.
3. Results and Discussions
3.1. Alginate Diet Effect on Weight
- Based on the results of the study with 25 mice, both alginate diet of brown seaweed Sargassum and Padina maintenance for 90 days, showed no toxic effect on the physiological and biochemical conditions. Before treatment, there was no significant difference in body weight of rats due to a homogeneous sample. Overall both control mice and mice that were given feed containing alginate, are in good condition and there were no deaths. The behaviour of all the mice in the control and four treatment looks healthy, active, and there are no signs of poisoning. All mice that fed a diet containing alginate of both these types, showed no effect on their body weight. This is in accordance with another research that if the repeated administration of a particular substance with the highest or the maximum dose and showed no signs of toxic or abnormality, it can be said that the substance is safe for long term use [6]. There is no weight loss at all Wistar mice were given feed containing alginate. But it has increased body weight, with a mean increase of 49-60 grams (Figure 1).
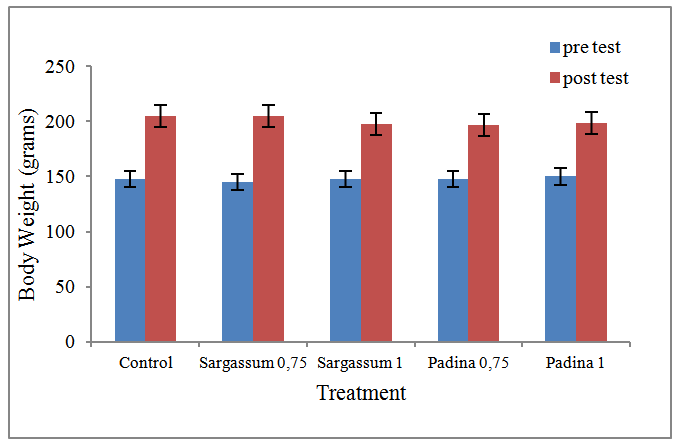 | Figure 1. Effect of treatment by alginate from Sargassum and Padina on body weight of wistar mice at the pre and post test |
3.2. Alginate Diet Effects on Levels of ALT and AST
- Tests on blood and serum biochemical testing of liver cell damage due to the possibility of a toxic effect, carried out tests on the levels of SGPT and SGOT enzymes, which can be seen in Figure 2.
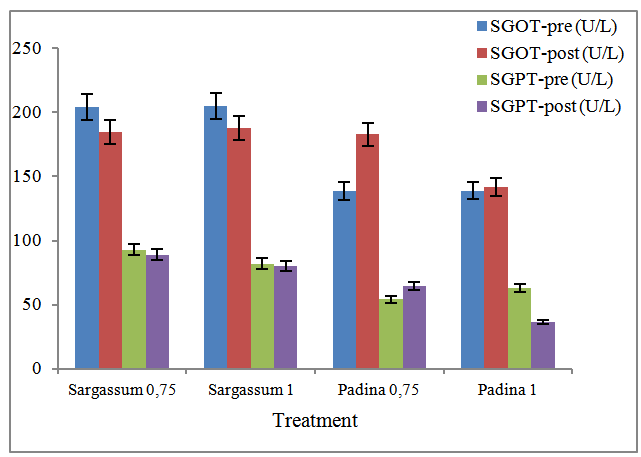 | Figure 2. Levels of SGOT and SGPT of wistar mice |
4. Conclusions
- Based on the results of a study of the levels of SGPT and SGOT of Wistar rats, it was concluded that alginate from Sargassum at a dose of 0.75% and 1% did not give a real effect on the occurrence of liver cell damage, with evidence of ALT and AST levels at the end of treatment is lower than the control. Diet alginate Padina types have a tendency to result in damage to the liver cells with doses ranging from 0.75%.
ACNOKLEDGEMENTS
- The authors thank to Directorate of Research and Community Service, Kopertis 7, East Java Republic of Indonesia 2014.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML