-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2014; 4(4): 185-192
doi:10.5923/j.fph.20140404.02
The Assessment of Some Biochemical and Immunological Effects by Amphetamine and Orlistat on Obesity in Rats
Hanan Mohamed Amin Shalaby, Nagy Sadek Tawfek, Bahaa Kenawy Abo-El Hussein, Marwa Safaa El-Din Mohamed Abd El-Ghany
Department of Zoology, Faculty of Science, El-Minia University, Egypt
Correspondence to: Marwa Safaa El-Din Mohamed Abd E, Department of Zoology, Faculty of Science, El-Minia University, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Obesity is a diseaseinvolving body weight gain. Several syntheticdrugs of better efficacy are being introducedin the modern system of medicine. Orlistatis a pharmacological agent promoting weightloss in obese subjects via inhibiting of gastricand pancreatic lipase. Amphetamine is potent psychostimulants and commonly used drugs of abuse. To evaluate the effect of amphetamine and orlistat on rats fed high fat diet. Forty male Albino weighing (180-200 g) strain were fed on high fat diet for six week to induce obesity. The obesity rats were randomly classified into 5 groups (8 rats each) and treated with orlistat and amphetamine and mixture of them for six week. Treatment with both amphetamine and orlistat had significant effect in reducing body weight, besides, supplementing diet with orlistat increase food intake. Both amphetamine and orlistat had the ability to reduce lipid profile such as t-cholesterol, triglyceride, HDL and LDL. While amphetamine and orlistat had the ability to increase ALT, AST, and ApoA1 compared with normal group. Orlistat decreased AFP and amphetamine increased AFP compared with HFD group, amphetamine treatment did not alter either total bilirubin or pancreatic lipase activity while orlistat clearly reduced their concentration. Treatment with both amphetamine and orlistat had significant effect in reducing SOD, GSH, and catalase activity. Orlistat supplementation increased a significant ghrelin level while orlistat decreased leptin compared with HFD group. Treatment with both amphetamine and orlistat had significant effect in increasing insulin and glucose. Orlistat has a great ability to reduce body weight with inhibiting pancreatic lipase level and decreased lipid profile while amphetamine increase pancreatic lipase.
Keywords: Obesity, Orlistat, Amphetamine, Pancreatic lipase, Leptin and ghrelin
Cite this paper: Hanan Mohamed Amin Shalaby, Nagy Sadek Tawfek, Bahaa Kenawy Abo-El Hussein, Marwa Safaa El-Din Mohamed Abd El-Ghany, The Assessment of Some Biochemical and Immunological Effects by Amphetamine and Orlistat on Obesity in Rats, Food and Public Health, Vol. 4 No. 4, 2014, pp. 185-192. doi: 10.5923/j.fph.20140404.02.
1. Introduction
- Overweight and obesity have been important public health problems throughout the world, affecting both developed societies and developing countries. The World Health.Organization estimates that there are currently more than 400 million obese and more than 1.6 billion overweight adults, a number that is expected to double by 2015 [1]. Considerable evidence indicates that obesity is associated with numerous diseases and metabolic abnormalities, such as type 2 diabetes, hypertension, dyslipidemia, coronary heart disease, and certain cancers [2, 3].Obesity is a disease involving body fat storage and body weight gain. The recent health crisis has spurred research in weight control, including studies in diet, exercise, surgery and pharmaceutical preparations. Because obesity is caused by a build-up of fat in the body due to, for example, the over-consumption of high fat food, modern therapeutic approaches are mostly focused on blocking or stimulating various biomolecules and enzymes involved in fat metabolism [4].Obesity, which describes the condition of abnormal accumulation of body fat mass, is directly related to increased risk of several chronic diseases, including glucose intolerance, cardiovascular disease hypertension, hyperlipidaemia, hemostatic variables and increased insulin resistance [5].It has been shown that a modest reduction (5%-10%) of body weight lowers cardiovascular disease risk factor; instigates modest improvements in blood pressure and serum cholesterol; and reduces the incidence of type-2 diabetes, markers of endothelial function, and inflammatory signatures [6].It is generally accepted that the tremendous rise in the obesity prevalence across the globe is driven primarily by a combination of increased calorie intake and decreased physical activity, and strongly influenced by our genetic background.Consensus for obesity treatment is that clinical therapy should begin with lifestyle changes that focus on behavioral modification, diet, and exercise [7].Some drugs which had demonstrated positive weight loss potential such as amphetamine and orlistat.Amphetamine is a Drug used to induce weight loss may reduce appetite or increase satiety, reduce the absorption of nutrients, or increase energy expenditure. Weight loss with pharmacotherapies is generally modest, that is, usually 2 to 7.9 kg more than that achieved with placebo treatment [8].Orlistat is a pharmacological agent promoting weight loss in obese subjects via inhibiting of gastric and pancreatic lipase, an enzyme that is crucial for the digestion of the long chain triglycerides, which at a three daily dose of 120 mg reduces fat absorption by 30% and has been proven to be useful in facilitating both weight loss and weight maintenance [9].Nevertheless, it is not known if orlistat has any impact on the clinical outcomes of other diseases and its long term safety is still to be determined [10] Natural products and their active principles, as sources for new drug discovery and treatment of diseases, have attracted attention in recent years.
2. Material and Method
- The experiment was conducted on (n = 40) adult male albino rats with average weight 180 - 200 g) strain were fed on high fat diet for six week to induce obesity. The obesity rats were randomly classified into 5 groups (8 rats each) and treated with orlistat and amphetamine and mixture of them for six week. Rooms (25°C) with constant humidity and 12h/12h light/dark cycle prior to experimental Protocols. Food and water were supplied ad libitum. The experiment was designed to be as painless and harmless as possible.Animal groups The animals were housed in temperature controlled were divided as follow:Group 1 (G1): served as the normal control group. Rats were fed balanced diet and received no intervention.Group 2 (G2): the included rats were fed high fat diet for six weeks, obesity Control group. Also, saline solution was administered i.p to the rat of this group 3 months.Group 3 (G3): included the group which was treated with orlistat group. Rats were fed high fat diet and received (12 mg/kg) of orlistat by i.p. injection dissolved with saline (SAL) (1ml/kg) [11].Group 4 (G4): included this group that was treated with amphetamine group. At the same period like orlistat group. The animals received amphetamine in a dose of (1.5 mg/kg) by i.p injection dissolved with saline (1mg/kg) [12].Group 5 (G5): this group received both orlistat and amphetamine in different doses from the other groups (6 mg/kg orlistat), (0.75 mg/kg amphetamine) for the same duration like other treated groups.The composition of balanced diet and high fat diet was as follows:Balanced diet for feeding normal control rats: 10 g protein, 10g fat, 74.4 g carbohydrates, 3.5 g mineral, 1 g vitamin, 0.1g methionine and 1 g fiber9 [13].High fat diet for induction of obesity: 10 g protein, 30g fat, 54.4 g carbohydrates, 3.5 g mineral, 1 g vitamin, 0.1g methionine and 1 g fiber [14].Body Weight Gain and Food ConsumptionIndividual body weight gains were recorded before study imitation (Day 0), and weekly thereafter. Mean body weight gains were calculated for each group at each interval and for the overall testing interval. During the study, food consumptions were measured weekly per cage and mean food consumption by individual rat were calculated. At the end of the experimental period, animals were fasted overnight but allowed free access to water. Animals were also weight immediately prior to sacrifice (fasted body weight). Animals were sacrified under anesthesia with diethyl ether, and then blood was collected and refrigerated for one hour to clot. Samples were then centrifuged for 5 minutes.Biochemical AnalysisBlood samples were immediately collected in clean and dried Wiesserman tubes from the portal vein. First part of blood was collected in tubes containing potassium oxalate and sodium fluoride for the estimation of glucose by O-toluidine method [15]. Second part of blood was left to coagulate then centrifuged at 3000 rpm for 15 minutes to obtain serum. Serum insulin and leptin were estimated according to [16] and [17].Serum cholesterol, triglycerides (TG), high density lipoprotein cholesterol (HDL-c), and total lipids were determined by using enzymatic colorimetric methods to [18]. Low density lipoprotein cholesterol (LDL-c) was calculated as following [LDL-C=Total cholesterol –HDL-C–VLDL-C] according to [19]. Serum alanine and aspartate aminotransferase (ALT&AST) activities enzymes were estimated according to [20]. Blood superoxide dismutase (SOD) catalase was estimated according to [21]. Lipid peroxides values were determined with spectrophotometric measurement of the amount of malondialdehyde equivalents with thiobarbituric acid and was expressed as thiobarbituric acid reactive substances (TBARS: n mol malondialdehyde / mg protein) [22]. Glutathione reductase (GR) [23] Serum total bilirubin was determined according to the method by [24].The determination of pancreatic lipase activity was performed according to previously described method [25] Apolipoprotein, APOA1, was quantified by ELISA using specific polyclonal antibodies as previously described [26] ELISA kits for detecting rat serum ghrelin were purchased from Abcam Biochemicals, USA [27].Statistical AnalysisCollected data were presented as mean SD and statistically analyzed using one way analysis of variance (ANOVA). Student "t" test was used for significance according to [28].
3. Results
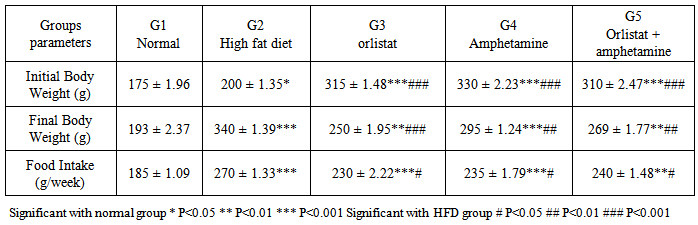
- Gain in Body Weight and Feed Efficiency RatioTable 1 shows the initial and final body weights (g) and food intake/week after feeding rats for 45 days with control diets, high fat diets, and high fat diet supplemented with orlistat, or amphetamine. On average, the rats of G1 and G2 gained weight throughout the experimental period, when compared to the initial body weight, while significant decrease is observed in G3, G4 and G5, when compared to G2. Besides, there was a significant increase of food intake in HFD group when compared to normal groups, while significant decrease is observed in G3, G4 and G5, when compared to G2.
|
|
|
|
|
|
4. Discussion
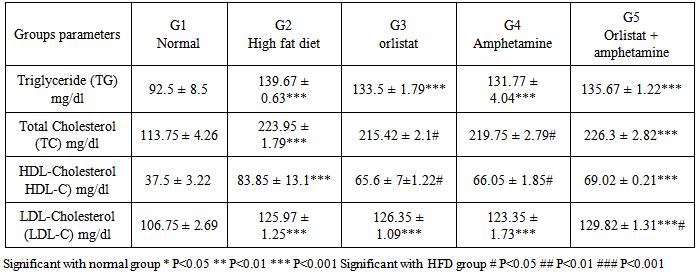
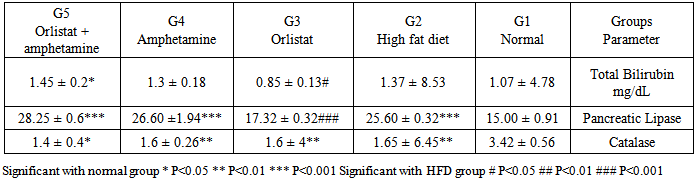
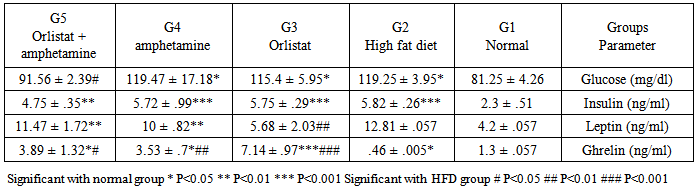
- Obesity, defined as a BMI >30 kg/m2, is a global epidemic that currently affects over 185 million adults in industrialized nations, 115 million in the developing world and over 18 million children under the age of five. Patients who have a BMI >25 are considered overweight, while a BMI >30 is considered obese, and a BMI >40 is considered morbidly obese. In the US, more than 27% of the population is currently obese, with over half of the population being overweight. There are approximately 260,000 to 380,000 deaths a year from factors related to obesity in the US [29].Our results demonstrated that the rats in normal (G1) and high fat diet (G2) groups gained weight throughout the experimental period, when compared to the initial body weight, while significant decrease was observed in orlistat group (G3), amphetamine group (G4) and orlistat and amphetamine group (G5) when compared to G2. Besides, there was a significant increase of food intake in HFD group when compared to normal groups, while significant decrease is observed in G3, G4 and G5, when compared to G2. However, obesity is defined as an increase in mass of adipose tissue, confers a higher risk for metabolic diseases such as non-insulin-dependent diabetes, cardiovascular disease, and stroke and an increased incidence of morbidity [30]. Previous study showed that orlistat is minimally (<1%) absorbed from the gastrointestinal tract, promotes significant weight loss when used in conjunction with a mildly hypoenergetic diet, and lowers blood lipid concentrations [31]. Results of the serum pancreatic lipase levels showed that there was reduction in G3 comparing with G2, but no significant effects were noticed in G4 and G5 comparable to G2. On the other hand, there was signifcant increase in the enzyme level recorded in G4 and G5 when compared to the normal group. These results indicate that amphetamine has no positive effect on pancreatic lipase level. On the other hand, it was reported that, orlistat is the only approved inhibitor of the gastrointestinal lipases, predominantly pancreatic lipase, necessary for the hydrolysis of triglyceride to free fatty acids in the lumen of the gut. Because this agent can reduce the absorption of dietary fat by up to 30%, it produces weight loss comparable to or greater than that obtained by placing an individual on a fat-restricted diet [32]. Higher values of serum lipid profile were observed in G2 when compared to the G1. There was a significant reduction in TG, TC, HDL-C levels in G3, G4 and G5 when compared to G2, while non-significant reduction in LDL-C was observed in G3, G4 and G5 when compared to G2. On the other hand, comparing results of HDL-C level and LDL-C level between G3 and G4 showed that there was significant increase in HDL-C level in G4 while opposite results seen in LDL-C level. While there was a significant reduction in TG level and total cholesterol in G3 when compared to G4 respectively. These results indicate that orlistat has better ameliorating effect on triglycerides and total cholestrol and amphetamine administration is better for HDL-C and LDL-C levels and administration of each of them alone is better than the two in combination. However, it could be observed that, treating obese rats with Orlistat, and amphetamine improved the lipid fractions. It was reported that, improvement in concentrations of cholesterol and triacylglycerols resulted from therapy with orlistat is a result of its effect on the body's ability to absorb dietary fats; orlistat is known to be associated with an increased incidence of gastrointestinal events in its users [33]. Orlistat is a gastric and pancreatic lipase inhibitor that alters energy balance by reducing the absorption of triglyceride and cholesterol from the gastrointestinal tract [34]. Reduction of the enzymatic activity is mediated through the covalent binding of orlistat to the serine residue of the lipase active site [35] There was a great relation between lipases and plasma TG levels, in that the triacylglyc-erols absorption efficiency is one of the main factors contributing to the plasma TG level; however, the dietary TG are not absorbed as much until hydrolyzed to fatty acids by triacyl-glycerol lipases. Pancreatic lipase is involved in the TG absorption from the small intestine to the enterocytes and if somehow this initial move- ment of TG from the small intestinal lumen is blocked, hyperlipidemia can be prevented. Thus, an inhibitor of digestive lipase that helps to limit intestinal fat absorption could be proved as useful medication for the treatment of hyper- lipidemia and holds great promise as an anti- obesity agent. In adults, orlistat, at the standard dose of 120 mg three times daily, inhibits 30% of triglyceride absorption. In placebo-controlled studies, adults treated with orlistat for periods as long as 2 years exhibited greater average weight loss, better weight maintenance, lower total and low density lipoprotein-cholesterol and improved glycemic control for patients with type 2 diabetes [36]. Also, it was reported that, during weight loss, orlistat reduced fat absorption, as shown by a decrease in serum LDL cholesterol that was expected, and that corresponds to an 25% decrease in cholesterol absorption and a 30% decrease in fat absorption [37].Amphetamine treatment did not induce any significant effect on pancreatic lipase level as observed in G4. The presence of lipase inhibitors have been reported in some natural sources and aqueous extracts of some medicinal herbs [38]. Plants were screened for their antilipase activity using ra- dioactive method, the study found that many plants can improve body weights and did not possess any lipase inhibitor effect. This means that amphetamine may able to reduce lipid profile without alterations in pancreatic lipase level [39].Results of the present study showed difference in the total bile content of rats in all treated groups. There was non-significant increase in the total content of bilirubin in G2 comparing with G1. On the other hand, orlistat group was significantly lower than all experimental groups, but G4 and G5 have non –significant effect comparing with G2. It was hypothesized that orlistat treatment decreases plasma bilirubin concentration in rats by increasing turnover and fecal excretion of bilirubin [40] Clearly, amphetamine treatment did not induce any significant effect in total bilirubin in G4 and G5 when com- pared to control and normal group.Analysis of catalase activity (CAT) in serum revealed that, the total activity of this enzyme was reduced in G2 compared with G1. On the other hand, there was no effect in the result of G3, G4 and G5 comparing with G2. These results indicate that there is no positive effect of orlistat, amphetamine or the two in combination on catalase activity. It was clear that constant generation of prooxidants, including oxygen free radicals, is an essential attribute of aerobic life [41]. The high level of oxidative stress associated with the increased lipid peroxidation may be one of the reasons why those who are overweight are at greater risk for developing heart disease [42]. Table (3) reveals that feeding with G2 resulted in significant increase in AFP (alpha phetoprotein) and APO A1 (Apo lipoprotein A1) in rats as compared to rats fed with G1 (p≤0.05). G3 and G4 showed significant in serum AFP level as compared to G2,but G5 non significant compare with G2 .The rat groups which were treated with G3 ,G4 and G5 showed non significant in the values of serum APO A1 (p<0.05, 0.01&p<0.001) compared with G2.The present data indicated that high fat diet increased serum AFP, APO A-1, AST and ALT enzymes significantly as compared with G1. Treatment with orlistate showed non –significant decrease in the previous parameters compared with G2. On the other hand, all other treated groups showed significant decreasing effect on these parameters indicating positive ameliorating effect of amphetamine. Amphetamine may have antioxidant effects that could be beneficial in oxidative stress and may play a role in liver injury by inducing synthesis of glutathione, an important mediator against hepatocellular injury. Data presented in table (5) showed that G2 had significant redution in the values of serum SOD, GSH, and increase in L.PEROX compared with the normal group. Treatment of rats with orlistat and amphetamine showed improvement in serum SOD and GSH, instead of being non-significant, supposing antioxidant effect for these agents. Administration of orlistat and amphetamine together did not have any effect. Conversely, the two agents in combination had a potent effect on serum L.PEROX. Cells have different antioxidant systems such as glutathione and various antioxidant enzymes to protect various tissues from free radicals attacks. Apart from glutathione, the antioxidant enzymes including SOD, CAT and GSH dependent enzymes such as glutathione peroxidase (GPX), and glutathione transferase (GST) may minimize or remove the oxygen radical cascade and reduce cytotoxic oxidative damage in cell [43, 44]. Administration of high fat diet caused hyperglycemia and hyperinsulinemia in rats of G2 as compared to G1. Treatment with orlistat and amphetamine in G3 and G4 showed non-significant decrease in serum glucose and insulin levels as compared to G2, but, there was significant decrease in G5 in these parameters comparing with G2. So, indicating better effect for the mixture. In this respect, previous study reported that, the addition or orlislat to a conventional weight loss regimen significantly improved oral glucose tolerance and diminished the rate or progression to the development of impaired glucose tolerance and type 2 diabetes. Obesity increase incidence of obesity-related disorders and cardiovascular diseases. Obesity is a disorder of energy balance and is associated with hyperinsulinemia, insulin resistance, and abnormalities in lipid metabolism. In addition, hyperinsulinemia and insulin-resistance contribute to vascular dysfunction, because the opposing endothelium - dependent vasodilating and vasoconstrictor effects of insulin are shifted toward a predominant vasoconstriction in patients with obesity. The present study showed that the serum leptin and ghrelin have non-significant decrease in response to high fat diet as compared with G1. Conversely, serum ghrelin were increased significantly in G3, G4 and G5 as compared with G2. Leptin has been shown in many studies to inhibit insulin release [45] It has been reported that leptin is produced by adipose tissue to signal fat storage reserves in the body, and mediates long-term appetitive controls (to eat more when fat storages are low and less when fat storages are high). Leptin participates in the modulation of energy [46, 47]. We can conclude that both orlistat and amphetamine can modify high fat diet–induced obesity. However, the effect of orlistat is more potent and exert its effect by inhibiting pancreatic lipase.Also, serum leptin increased significantly in obese rats in response to HFD, Leptin plasma concentration and mRNA expression in adipose tissue are directly related to obesity severity, as an increase of fat mass is associated with an increase of leptin which makes leptin an indicator of the total fat mass (Lopez et al-48. 2005) [48]. Also, our results showed that serum ghrelin decreased significantly in obese rats as compared with the control non obese rats, these results were in agreement with Tschöp et al-49. (2001) [49].In the present work we investigated the effect of orlistat treatment and amphetamine treatment with HFD on the serum lipids, leptin and ghrelin as well as the inflammatory cytokines. Both orlistat and amphetamine reduced the body weight, adipse tissues and serum lipids as compared with the HFD fed rats. Orlistat is a reversible lipase inhibitor that acts by inhibiting the absorption of dietary fats, while amphetamine increased lipase. Consequently with decreased body weight and total adipose tissue levels, the serum level of the inflammatory markers reduced significantly as compared with HFD fed rats. Also, serum leptin decreased in response to orlistat treatment and amphetamine with significant increase in the serum ghrelin level.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML