-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2014; 4(3): 140-150
doi:10.5923/j.fph.20140403.09
Extraction Process Modification to Enhance Properties of Skin Gelatin of Pangas Catfish (Pangasius pangasius)
Ratnasari I.1, 2, Sudarminto S. Y.3, Nusyam H.4, Simon B. Widjanarko3
1Postgraduate Program of Agricultural Science, Faculty of Agriculture, University of Brawijaya, Malang, East Java of Indonesia
2Department of Fishery Product Technology, Faculty of Agriculture, Palangka Raya University, Palangka Raya, Central Kalimantan (73111A), Indonesia
3Department of Agricultural Product Technology, Faculty of Agriculture Technology, Brawijaya University, Malang, 65145, Indonesia
4Department of Fishery Product Technology, Faculty of Fisheries and Marine Science, Brawijaya University, Malang, 65145, Indonesia
Correspondence to: Ratnasari I., Postgraduate Program of Agricultural Science, Faculty of Agriculture, University of Brawijaya, Malang, East Java of Indonesia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
This study was aimed at studying the effect of preliminary treatment variations on the pangas catfish (Pangasius pangasius) skin gelatin, particularly the gel strength. The preliminary treatment using the whiting solution gave higher gelatin property (P<0.05) than that using alum (Al3(SO4)2) and Calcium Hydroxide (Ca(OH)2). High gelatin (dry basis) was 23.37% and gel strength was 360.18 g. The high viscosity, gelling temperature, and melting temperature were 7.87 (cP), 18.67℃, and 31.67℃, respectively. The gelatin SDS-PAGE with pre-treatment of whiting (CaO) was 136.56 kDa, and the rheological test exhibited higher elastic modulus (G’) and viscous modulus (G”) than that of other treatments and commercial gelatin. SEM micrographs also showed that the catfish gelatin pre-treated with the whiting had thick strand with small voids and dense tissue. As conclusion, the extraction process modification using the whiting could increase the skin gelatin properties of the pangas catfish (Pangasius pangasius), and the fish skin is a prospective source of good gelatin with desired functional properties.
Keywords: Modification, Pre-treatment, Fish Gelatin, Pangasius pangasius, Rheology, Gel strength
Cite this paper: Ratnasari I., Sudarminto S. Y., Nusyam H., Simon B. Widjanarko, Extraction Process Modification to Enhance Properties of Skin Gelatin of Pangas Catfish (Pangasius pangasius), Food and Public Health, Vol. 4 No. 3, 2014, pp. 140-150. doi: 10.5923/j.fph.20140403.09.
Article Outline
1. Introduction
- Gelatin is a fibrous protein produced through thermal denaturation or collagen partial degradation of animal bone and skin [1]. Gelatin is mainly used in food, pharmaceutical, medical, cosmetic and photographic industries and has unique physical and chemical properties [2]. It is mostly applied as stabilizer, gelling, fastener, emulsifier, adhesive, and edible food wrapping. It could also be used for diabe- tics and can reduce body weight [2] [3]. In food industry, gelatin is one of the water-soluble polymers that can be used as materials to raise the food elasticity, consistence, and stability [4]. Gelatin quality is mostly dependent upon the rheological properties, particularly the gel strength and viscosity, but other characteristics, especially transparence, presence of color and smell and solubility, are also important [5]. For specific applications, it is highly dependent upon its physico-chemical features that are highly affected by species and tissue extracted and extraction method [1]. Moreover, the extraction process of gelatin influences the gelatin properties, and the extraction efficiency of the gelatin is dependent upon the extraction method where the collagen is pre-treated [6]. Gelatin extraction process from fish skin is commonly done using acids or alkali in order to result in desired properties [7][8]. The use of acids or alkali in the past was done by Muyonga [9][1][10][11] producing lower gelatin gel properties than those of commercial gelatin. Gelatin quality depends on the physical, chemical and structural characteristics, but the nost important physical properties are gel strength and viscosity [2]. It is measured from gel strength or bloom values that could be classified as low bloom (< 150 g), medium bloom (150-220 g) and high bloom (220-300 g), respectively [12]. Study on fish skin gelatin showed that fish gelatin had lower gelatin gel properties than animal gelatin beside melting and gelling temperature [13]. Gel strength of animal gelatin is 200-300 g and melting temperature is above 30℃ [3]. Although fish skin gelatin properties are different from those of mammals and fowls, the fish skin gelatin has benefits using numerous fish wastes and can be spared from mad cow disease (bovine spongiform enchephalopathy/BSE) and Foot and Mouth/FMD disease [14]. The gel properties of freshwater gelatin are better if compared with those of marine fish. The freshwater fish gelatin gel is rather similar to that of mammal bone and skin [15] [16].Pangas catfish are freshwater fish often directly consumed or processed. They are a promising raw material source for gelatin extraction [17]. They are also easily cultured, relatively big sized, their flesh could be filleted, have a lot of skin, and collagen-rich gelatin source. Freshwater fish like pangas catfish are one of gelatin sources whose potency needs to be developed. As gelatin extraction raw materials, the pangas catfish skin can increase the byproducts and solve some home industrial wastes from fish processing. The pangas catfish skin can be obtained fish fillet industrial wastes and home industry of cracker processing. Pangas catfish skin gelatin has lower gelatin properties than those of cow gelatin [17], and therefore, efforts are needed to increase the pangas catfish (Pangasius pangasius) skin gelatin properties to be able to use in extensive applications. Several efforts carried out were modification with enzim transglutamine enzym [18], gel property modification through polysacharide addition [19], addition of salt solution, such as NaCl, KCl, MgCl2 and MgSO4 [20], pre-treatment of sturgeon (Acipenser baeri) skin through addition of alkali and acetic acid (HAC) solution [1], 14 days of whiting solution [21], bleaching with hydrogen peroxide (H2O2) [22], ultra-high presurre (UHP) pre-treatment with hydrocloric acid [23], and chemical modification with genipin, glutaraldehyde and caffeic acid [24]. Nevertheless, information on gelatin extraction process modification through pre-treatment of whiting, Ca(OH)2 and alum has not been gained that high gelatin properties of pangas catfish skin, particularly gel strength, could be obtained to be used for various types of products and as a range in food material applications.The gelatin application as gelling material in food processing is limited based on its gel properties, particularly the gel strength. For extensive applications, a study on gelatin extraction process modification of the pangas catfish skin with pre-treatment of whiting, alum and Ca(OH)2 solution needs to be done. Our study showed that the pangas catfish skin gelatin had lower gel strength, viscosity, gelling temperature, and melting temperature than the commercial one [17], so that the quality of the skin gelatin properties, especially gel strength, needs to be increased for extensive applications. This study was aimed at knowing the effect of gelatin extraction process using the pre-treatment of whiting (CaO), alum (Al2(SO4)3) and calcium hydroxide (Ca(OH)2) on the pangas catfish skin gelatin properties in order to be able to produce high gel strength so that it could be used for various kinds of product applications and could gain basic information on extraction process method with correct pre-treatment variations usable as a range in food material applications.
2. Materials and Method
2.1. Materials
- Pangas catfish (Pangasius pangasius) of approximately 600-700 g body weight was collected from local fish sellers in Palangka Raya, Central Kalimantan. The fish skin was manually taken and washed. It was then laced in the polyethylene bags and stored at -20℃ up to use. Other materials used were commercial powder gelatin (G.merck, D.6100 Darmstandt F.R Germany), whiting (CaO) and alum (Al2(SO4)3) obtained from in traditional market, and calcium hydroxide (Ca(OH)2) and citric acid (merk) from chemicals store.
2.2. Method
2.2.1. Experimental Design
- The experiment was carried out using Complete Randomized Design with three treatments, (i) whiting, (ii) Calcium Hydroxide, and (iii) alum. It was done in the laboratory of Agricultural Product Technology, Brawijaya University, Malang. The gelatin sample was analyzed for (i) gel strength, (ii) viscosity, (iii) gelling temperature, (iv) melting temperature, (v) pH, (vi) SDS-PAGE, (vii) Rheology, and (viii) SEM.
2.2.2. Pre-treatment
- Fish skin was thawed and washed, cut by 1 x 1 cm and rewashed. Before extraction process using citric acid, 100 g fish skin was extracted using whiting (CaO), alum (Al2(SO4)3) and calcium hydroxide (Ca(OH)2) each of which were for 1 hour. The skin was then washed in running water until neutral pH was found and drained.
2.2.3. Citric acid-skin Extraction
- The fish skin previously extracted with pre-treatment of whiting (CaO), alum (Al2(SO4)3) and calcium hydroxide (Ca(OH)2) for 1 hour was extracted with 1% citric acid (1:3 b/v) of pH 3 for 12 hours. It was then washed 6 times until neutral pH (pH 6 – 7) was reached. It was extracted with water in the water-bath at 60℃ for 6 hours. The gelatin solution was filtered through cloth and then Watman no.1 filter paper. It was then cooled until gel gelatin was formed. It was dried in Cabinet Dryier at 60℃ for 24 hours. The dry gelatin was refined and filtered in mesh 60 to obtain gelatin powder.
2.3. Analysis
2.3.1. Yield of Gelatin [25]
- Gelatin production was calculated as % yield (wet weight basis) = dry weight of gelatin/ wet weight of skin x 100 %. The gelatin extracts of each pre-treatment variation was determined by gelatin weight comparison with fish skin weight. The physical performance, i.e. color and gelatin gel properties, was determined from physical observations.
2.3.2. Determination of Gel Strength [26]
- Gelatin was solved in aquadest at 60℃ to get 6.67% (w/v) gelatin solution concentration. It was stirred using a magnetic stirrer up to homogenous, poured in Standard bloom jars (3 cm diameter and 2.7 cm high), left for 2 minutes, cooled in the refrigerator at 10℃ for 16-18 hours so that gel was formed. The gel strength was measured with a Tensile strength Instrument (Digital Force Gause model Imada/ZP-200N), with load cell of 5 kN and 1 mm diameter- flat teflon cylindrical surface. The probe speed was 0.5 mm/s at the depth of 4 mm. The gel strength (maximum strength) was expressed in gram force.
2.3.3. Determination of Viscosity [25]
- Gelatin solution of 6.67% concentration was heated in a boiling waterbath while regularly stirred until the temperature was 60℃. Viscosity was measured with a viscometer brookfield. Spindle was previously heated at 60℃ and then connected to the viscometer brookfield. Its position in the hot solution was set to the proper heat. The viscometer was turned on and the solution temperature was measured. When the solution temperature reached 60℃ and the viscosity was known at the reading scale of 1 to 100. The reading was done after 2 full 1 minute rotations for no. 1 spindle.
2.3.4. pH [27]
- pH was measured with glass electrode (Toledo MPC 227 pH meter, Mettler-Toledo GmbH, Schwerzenbach, Switzerland) after the pH-meter had been standardized at pH 4.0 – 7.0, and the pH value on the screen was recorded. The gelatin solution pH was measured using British Standard Institution method, BSI 759 [28]. For the isoelectrical pH measurement, the gelatin solution was added buffer solution of pH 4 until precipitation occurred, and pH was measured when the precipitation started.
2.3.5. Determination Gelling Temperature and Melting Temperature
- 20 ml of gelatin solution from the extract was filled in the test tube and put into the cool box facilitated with thermometer. Crushed ice cubes were put little by little until gelatin gel was formed. Gelling temperature was taken at the time the gelatin solution became gel. The gelatin gel was then placed in the beeker glass, put in the waterbath, and heated at 40oC. The waterbath temperature was recorded using a thermometer since it was heated. The time of melting gelatin gel was taken as melting temperature.
2.3.6. Electrophoretic (SDS-PAGE) Analysis
- Molecular weight distribution of the gelatin extract was determined using SDS-polyacrylamine gel electrophoresis (SDS-PAGE). Gelatin sample was dissolved in distilled water at 60oC to make 1.5 mg/ml solution. It was then mixed with buffer sample (0.5 M Tris-HCL, pH 6.8, containing 5% SDS, 20% glycerol) in 1:2 ratio with the presence of 10% β-mercaptoethanol. The sample was heated at 100℃ for 2 minutes. SDS-PAGE was carried out with 7.5% gel following Laemmli [29]. After electrophorized, the gel was stained with Coomasie brilliant blue R250 dissolved in water, methanol and trichloracetic acid (5:4:1) and destained with methanol, distilled water and acetic acid-containing solution in 5:4:1 ratio and 15 μg protein was put in each well. High molecular weight markers (Sigma-Aldrich Chemical Co. USA) were used for gelatin molecular weight distribution estimation.
2.3.7. Rheological Test [22]
- Rheological measurements were carried out using DHR-1 rheometer (TA Instruments, Surrey, UK) with plate geometry (25 mm). Gelatin solution (6.67%w/v) was prepared by dissolving dry gelatin in 45℃-distilled water. The sample was measured in 1℃/min scan rate, 1 Hz frequency, 1500 pm gap and 3 Pa stress. The gelatin solution was firstly heated from 5℃ to 45℃, held at 45℃ for 2 min. and then cooled from 45℃ to 5℃. Elastic modulus (G’), viscous modulus (G”) and phase angle (rad) were recorded as temperature function. Melting and gelling temperatures were calculated by interpolation and taken as cross-over point of G’ and G” where tan δ became 1 and δ became 45° [30].
2.3.8. Scanning Electron Microscopy (SEM)
- Gelatin sample was put in ±10 mm holder. Non-conductive samples, such as organic, polymer, and others needed to be coated using Au-Pd (to make samples more conductive). The sample was put into the SEM chamber, pumped (High Vacuum or Low Vacuum) and after full vacuum, SEM/EDX (Merk FEI, Type Inspect S50) was ready to use (Beam On).
2.4. Statistical Analysis
- Data were collected based on the mean value of 3 measurements. The data were presented in mean value ± SD and P<0.05 was considered significant. Analysis variance (ANOVA) was done and mean comparison used Duncan’s Multiple range test applying Microsoft SPSS 17.0 for windows (SPSS Inc, Chicago, II, USA).
3. Results and Discussion
3.1. Yield of Extracted Gelatin
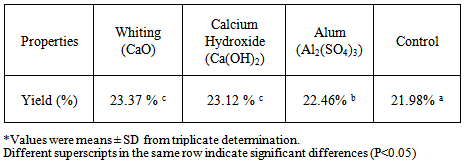
- Pangas catfish skin gelatin extract obtained from pre-treatment variations using whiting (CaO), alum (Al2(SO4)3), calcium carbonate (Ca(OH)2) and control solutions were 23.37%, 23.12%, 22.46% and 21.98%, sucessively (Table 1).
|
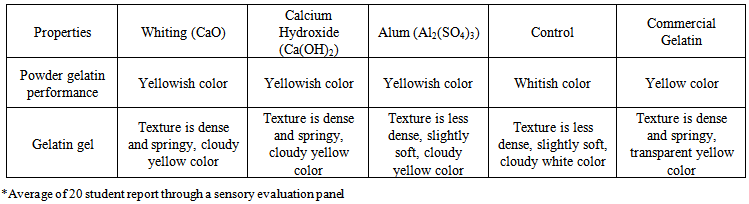 | Table 2. Visual description of Pangas catfish (Pangasius pangasius) skin gelatin with pre-treatment variations |
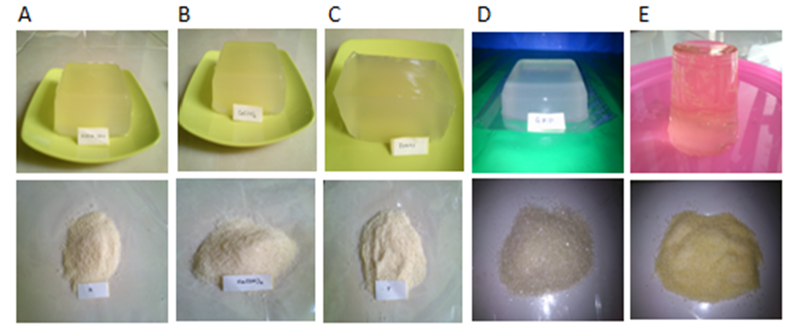 | Figure 1. Catfish skin gelatin gel and powder extracted with pre-treatment variations (A = whiting, B = Ca(OH)2, C = alum, D = control, E = Commercial gelatin) |
3.2. Determination of Gel Strength
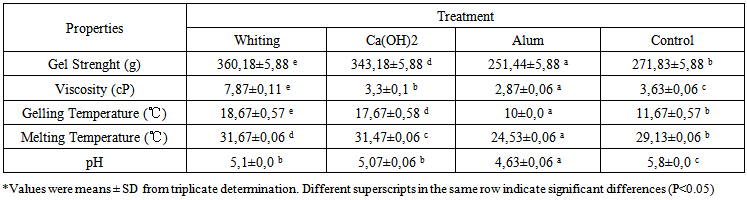
- Gel strength is one of the important functional properties of the gelatin. Present results indicated that the gel strength of fish gelatin sample from pre-treatment variations gave gelatin of different gel strength (P<0.05) (Table 3.). The gelatin gel strength of Pangas catfish pre-treated with whiting gave higher strength (360.18 g) than others, 343.18g, 251.44 g, 271.83 g, and 283.79 g, respectively, for calcium hydroxide, alum, control and commercial gelatin. The gel strength significantly increases (p<0.05) with pre-treatment variations, but there is no difference (p>0.05) in gel strength between whiting and calcium hydroxide pre-treatments. Whiting pre-treatment helps cleaning the non-collagen protein, could reduce collagen loss and skin swelling so that in each washing phase it could increase the gel strength. Hao [1] found that the pre-treatment of alkali and acid combinations could reduce the collagen loss, but it could significantly increase the gel strength and viscosity. This finding is similar to our present study that whiting pre-treatment could increase the gel strength and viscosity. Gel strength variation is related with acid composition and protein chain size [9], gelatin concentration and molecular weight distribution [35].
 | Table 3. Pangas catfish (Pangasius pangasius) skin gelatin properties with pre-treatment variations |
3.3. Determination of Viscosity
- Viscosity is the second important feature of gelatin [48]. The viscosity of the five samples studied shows significant difference (p<0.05) with a range of 2.87 – 7.87 cP (Table 3). The vicosity value (cP) of Pangas catfish gelatin with alum pre-treatment is lower than that of whiting, Ca(OH)2, control and commercial gelatin. Whereas the gelatin viscosity of whiting pre-treatment (7.87cP) is higher than that of commercial gelatin (3.93 cP). Increase in viscosity is followed by increase in strength gel, gelling temperature, melting temperature, and molecular weight distribution. Low gelatin viscosity from alum pre-treatment is related with low gel strength and molecular weight distribution observed. Viscosity is mostly controlled by molecular weight and molecular size distribution [49].Grossman and Bergman [50] reported that Tilapia skin had the gelatin viscosity of 5.1 cP, while skin gelatin viscosity was 7.70 cp recorded in red tilapia, 6.28 cp in walking catfish, and 8.21 cp in striped catfish [21], and the lowest viscosity (< 3.0 cp) was recorded in channel catfish gelatin [51]. Change in pH is known increasing the gelatin viscosity, and minimum gelatin viscosity occurred at the pH range of 6-8 [52]. This is consistent with the present study that high viscosity is followed with high pH. Other finding also found that higher gelatin proportion with large molecules (such as α-chains) had the highest viscosity [53]. Based on GMIA standard [45], the viscosity value of acidic gelatin is 1.5-7.5 cp for food grades, 4.4-4.5 cp for hard capsules, 2.5-3.5 cp for soft capsules, and 1.7-3.5 cp for tablet. Whereas the alkali gelatin has the viscosity range for food grade (2-7,5 cp), 4.5-6 cp for hard capsules, for soft capsules (3-4,5 cp) and 3-3.5 cp for tablets. Based on this standard, it could be concluded that gelatin from whiting pre-treatment with viscosity of 7.87 cp could be used for all applications, while those from calcium hydroxide, alum, control and commercial gelatin could be used for all applications, except for hard capsules, since their viscosity values are beyond the GMIA range.
3.4. Determination of Gelling Temperature and Melting Temperature
- Gelling temperature dan melting temperature of the gelatin with pre-treatment variation have sigfinificant effect (p<0.05) as shown in Table 3. Results showed that gelling temperature dan melting temperature of Pangas catfish pre-treated with whiting (18.67℃ and 31.67℃) were higher than those pre-treated with calcium hydroxide (17.67℃ and 31.47℃), alum (10℃ and 24.53℃), control (11.67℃ and 29.13℃) and commercial gelatin (15.67℃ and 31.4℃), but lower than those of commercial gelatin (15℃ and 34℃). Nagarajan [33] reported that the condition of gelatin extraction influenced the physico-chemicals of the gelatin, such as molecular weight distribution, number of β-chain and γ-chain components and free amino group content. Therefore, gelling temperature is not sufficiently affected by the extraction condition used in this study.According to Karim and Bhat [2], gellling temperature and melting temperature of fish gelatin ranged from 8 to 25℃ and 11 to28℃, respectively. Pranoto [54] found that gelatin extract of tilapia has melting temperature of 24.55℃, higher than pech tilapia (26.3℃) [9], but lower than cod (13.8℃) [55].Melting temperature of the gelatin from whiting and calcium hydroxide pre-treatment is higher (p<0.05) than alum pre-treatment, control and commercial gelatin. It could be correlated with high gel strength, viscosity and molecular weight distribution. Johnston-Barks [12] found that there is correlation between melting temperature and molecular weight distribution. Norland [56] and Gudmundsson [57] recorded melting temperature of 29.7 and 32.3℃ in bovine and porcine gelatin, respectively, but our present study through extraction process of pre-treatment variations (whiting, Ca(OH)2) recorded higher melting temperature than that of bovine. Difference in gelatin types could result in different physico-chemical characteristics influencing the thermal and rheological features, including melting temperature, gelling temperature and gel strength [41].
3.5. pH
- Gelatin pH values of Pangas catfish skin from pre-treatment variations and commercial gelatin reflecting significant difference (p<0.05) are presented in Table 3. Table 3 shows that the pH value of gelatin solution from whiting pre-treatment (pH 5.1) is lower than that of the commercial gelatin (pH 6.2) (p<0.05), and the pH from whiting pre-treatment is not significantly different (p>0.05) from that from Ca(OH)2. pre-treatment. The gelatin from alum pre-treatment has lower pH (4.63) than that from control gelatin, whiting pre-treatment, Ca(OH)2 and commercial gelatin. Low pH of gelatin from alum pre-treatment is consistent with low gel strength produced. This low pH value could result from that in extraction process, the skin collagen swelling has occurred so that in acid (citric acid) extraction process the skin swelling is developing, and as a consequence, it is not properly washed despite neutral washing water pH. Hao [1] stated that the pre-treatment with alkali and acid combinations could reduce the collagen loss, but it could also significantly increase the gel strength and viscosity, with a final pH around 5,0 was optimum for gelatin extraction. The changes in pH are known to influence the viscosity and minimum viscosity for gelatin has been observed in the pH range of 6-8 [52]. Based on the GMIA standard [45], the pH of acidic gelatin is 3.8-5.5 for food grade and 4.5-5.5 for hard capsules, soft capsules, and tablets, while the pH of alkali gelatin is 5-7.5 for food grade and 5.3-6.5 for hard capsules, soft capsules and tablets. It could be concluded that the gelatin from whiting and calcium hydroxide pre-treatments with pH of 5.1 and 5.07, respectively could be applied for food grade, hard capsules, soft capsules and tablets, while that from alum pre-treatment (pH 4.63) could only used in the range of acidic gelatin.
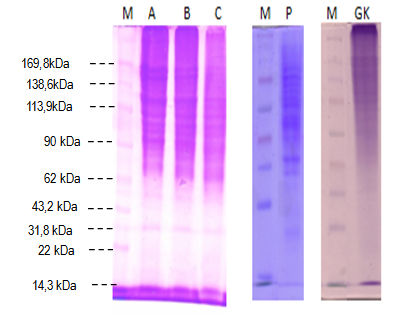
3.6. Electrophoretic (SDS-PAGE)
- Protein patterns of pangas catfish (Pangasius pangasius) skin extracted in pre-treatment variations are presented in Figure 2. The protein band of lane A (whiting solution pre-treatment) and lane B (Ca(OH)2 solution) is stronger than that in lane C (alum solution pre-treatment), control and commercial gelatin. Different effect between Whiting, Ca(OH)2 and alum could result from significant difference in the swelling process of Pangas catfish skin during the pre-treatment, in which, after the pre-treatment, the skin will swell, but whiting and Ca(OH)2 pre-treatment cause the skin less swell. Alum pre-treatment produces low collagen extracts due to much collagen loss in washing series. The alum pre-treatment gives weaker protein band (high molecular weight) than that of control (low molecular weight) (unpresented data). High molecular weight of alum is inconsistent with low gel strength, gelling temperature and melting temperature. This is related with pH difference of each solution (whiting, Ca(OH)2 and alum) causing different material collagen swelling process. It occurred in Alaskan pollock skin study [58]. Strong protein band in lane A (whiting solution pre-treatment) is consistent with high gel strength of pangas catfish skin gelatin, followed with high viscosity, gelling temperature and melting temperatures.Muyonga [9] reported that alkali and acid treatments are important to take out the undesired materials, such as non-collagen protein, by minimizing the collagen loss. The same yield was also previously reported for Alaskan pollock [58] [59] and Sturgeon [1] skins. Jongjareonrak [60] reported that low molecular weight of α-chain enables to cause protein degradation during the extraction. Degradation of the gelatin fragment is associated with low viscosity, melting point, high setting time and gel strength reduction.
3.7. Rheological Test
- Viscoelastisity of gelatin (6.67 g/l) solution was measured through heating and cooling program. Gelling and melting points could be seen from drastic decline of the cross-over point of G’ and G” where tan δ becomes 1 and δ become 45o [30]. The rheological test outcome of gelatin samples through heating (5-45℃) and cooling (45-5℃) scan is shown in Fig. 3 and 4. As a whole, the elastic modulus (G’) is larger than the viscous modulus (G”) in both heating and cooling scans.
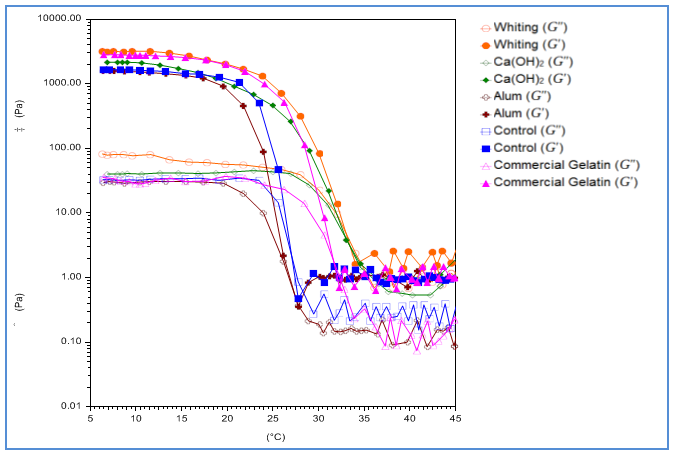 | Figure 3. Rheological heating scan (5-45oC) of catfish (pangasius pangasius) skin gelatin with pre-treatment variation. G’ = elastic modulus; G” = viscocity modulus |
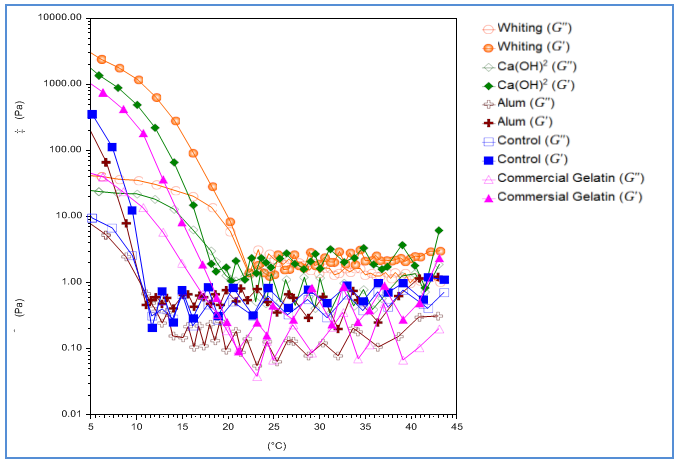 | Figure 4. Rheological cooling scan (45-5oC) of catfish (pangasius pangasius) skin gelatin with pre-treatment variation. G’ = elastic modulus; G” = viscocity modulus |
3.8. Scanning Electron Microscopy (SEM)
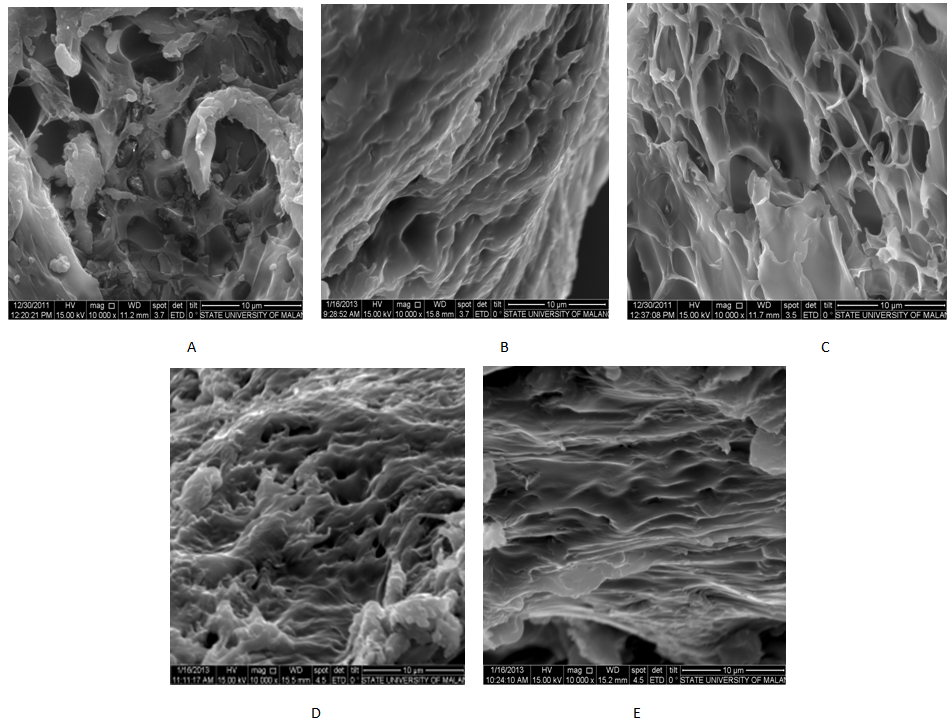
- Gelatin gel microstructures of commercial gelatin, whiting (CaO) pre-treatment, alum (Al2(SO4)3) pre-treatment and calcium hydroxide (Ca(OH)2) pre-treatment are resented in Fig. 5. All gelatin gels have spaces or sponges as in structures. In general, the arrangement and combination of protein molecules in the gel matrix contributes directly to the gelatin gel strength [26]. Interconnecting and dense tissues appear in the gelatin gel of whiting pre-treatment (Fig. 5.B). There is also thick strand seen with the best tissue of very small voids. The coarse and ununiform tissues with unclear strand appear in commercial gelatin (Fig. 5.A). The gelatin of calcium hydroxide pre-treatment has poorly uniform tissue with slightly thick strand and small voids and almost approaches to the gelatin gel microstructure of whiting pre-treatment. The control gelatin looks thinner and the are relatively large (Fig. 5.D). Microstructures of thin strands with ununiform tissues and large voids were seen in alum pre-treatment (Fig. 5.E). The gel with few inter-chain junctions or thin strands with loose tissues was easily disturbed by given power [26][40]. Therefore, pre-treatment varitions influence the gelatin gel which directly determines the gel properties (particularly the gel strength). It is known that the gel tissue microstructure is related with gelatin gel physical properties [51].
4. Conclusions
- The gelatin produced through extraction process modification of pre-treatment variations increased the gelatin properties of Pangas catfish skin. The pre-treatment of whiting and calcium hydroxide raised the gel strength, the viscosity, the gelling temperature, the melting temperature, SDS-PAGE and rheological test product, but better gel property was recorded in gelatin of whiting pre-treatment with high extraction production and gel properties. All gelatin under pre-treatment variations, but alum, exhibited higher gel strength than the commercial one. SEM micrographs of gelatin structure of Pangas catfish pre-treated with the whiting had thick strand with small voids and dense tissues.To obtain high extract and quality, this study recommends to do the extraction process using whiting pre-treatment followed with citric acid extraction. In addition, pangas catfish skin gelatin could be applied as new alternative source in product processing as substitute alternative of cow and pig gelatin.
ACKNOWLEDGEMENTS
- We are thankful to the University of Palangka Raya and Brawijaya University for providing convenience in using the experimental equipments and the Directorate General of Higher Education for the financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML