-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2014; 4(3): 110-122
doi:10.5923/j.fph.20140403.07
In vivo Effect of Essential Oils from Laurus Nobilis, Anethum Graveolens and Mentha Piperita on Mycobiota Associated with Domiati Cheese During Storage
Mady A. Ismail1, Al-Zahraa M. Darwish2, Nemmat A. Hussein1, Soumia M. Darwish3
1Department of Botany & Microbiology, Faculty of Science, Assiut University, Egypt
2Department of Dairy Science, Faculty of Agriculture, Assiut University, Egypt
3Department of Food Science and Technology, Faculty of Agriculture, Assiut University, Egypt
Correspondence to: Soumia M. Darwish, Department of Food Science and Technology, Faculty of Agriculture, Assiut University, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Three concentrations (3, 5, and 7 ml/100g retentate) of each of three natural oils were added during manufacturing of low salt white cheese. The mycobiota of cheese were assessed after 8 hours, 3 weeks, 7 weeks and 11 weeks. Twelve species were isolated (Aspergillusflavus, A. fumigatus, A. niger, A.versicolor, Penicilliumaurantiogriseum, P. camembertii, P. griseofulvum, P. islandicum, P. oxalicum, P. restrictum, Ulocladiumatrum and the yeast species Debaryomyceshansenii). The total counts of fungi increased in Laurusnobilisoil-treated cheese at the three concentrations after 3 weeks compared with control, but decreased after 7 weeks in treatment with 3 and 7% of oil concentrations. Cheese treated with Anethumgraveolens oil at 3% concentration showed the highest fungal counts after 7 weeks of storage. The fungal counts decreased by increasing all concentrations of Menthapiperita oil (3, 5 and 7%). Generally, treatment of cheese with M. piperita oil significantly decreased the total counts of fungi. On the other hand, oils of L. nobilis and A. graveolens at 3 to 7% concentrations caused an increase of total counts after 3 and 11 weeks but L. nobilis and A. graveolens oils caused a decrease after 7 weeks at 5 and 7% concentrations compared to control,. Isolates ofAspergillusflavus screened for aflatoxin production using Coconut agar medium (CAM) were positive foraflatoxin B production when observed at 365 nm UV light.
Keywords: Domiati cheese, Essential oils, Laurus nobilis, Anethum graveolens, Mentha piperita, GC-MS, Fungi, Aspergillus, Penicillium, Aflatoxin
Cite this paper: Mady A. Ismail, Al-Zahraa M. Darwish, Nemmat A. Hussein, Soumia M. Darwish, In vivo Effect of Essential Oils from Laurus Nobilis, Anethum Graveolens and Mentha Piperita on Mycobiota Associated with Domiati Cheese During Storage, Food and Public Health, Vol. 4 No. 3, 2014, pp. 110-122. doi: 10.5923/j.fph.20140403.07.
Article Outline
1. Introduction
- Cheese is highly nutritious as it provides protein, calcium, zinc and vitamins that are vital to good health (Kosikowski & Mistry, 1997). Domiati cheese is one of the most popular soft white cheeses in Egypt which is consumed fresh or after pickling for some months. This type of cheese is possibly made at home by small milk producers since it does not need complicated equipments. The pickled cheese could be stored at room temperature up to 6 months without deterioration (AbdEl-Kader, 1999). In Egypt, the salt content in cheese could reach up to 7 g salt/100 g (Ceylan et al., 2003; Elsanhoty et al., 2009). Salt (sodium chloride) is an important factor for two major roles: it acts as preserving agent and it contributes to the sensory (quality) of the cheese. Recently with increased prevalence of heart disease and blood pressure, increasing trend consumers to foods which was containing a small amount of salt so manufacturing tended to produce low salt products. In low salt content cheese, some microorganisms may grow and produce uncharacteristic visual appearance and diminish the commercial value of the cheese, and result in a reduction in quality and quantity (Soliman & Badeaa, 2002), therefore preservatives should be added to stop the activity of these microorganisms (Pintado et al. 2010).Additionally, fungi can grow in cheese and defect the color and aroma cheese (Erdogan et al., 2001).Growth of fungi causes spoilage and occurs sporadically on surface of hard cheese and packaged cheese (Hocking & Faedo, 1992). One of the modern ways to improve the hygienic safety of manufactured food products is to exploit the antimicrobial properties of natural plant extracts, allowing for the reduction of the use of preservatives, which constitute a potential human health hazard. Essential oils have been known for a long time and continue to be the subject of several studies that evaluate their microbial potential as alternatives to chemical agents in food industries (Djenane et al., 2012). Laurel (Laurus nobili L.) is rich in essential oil which is reported as toxic to many microorganisms (Hassiotis & Dina, 2011). Dill (Anethum graveolens L.) oil has also been reported to possess antibacterial (Rafii and Shahverdi, 2007) and antifungal drugs and food preservatives (Tianet al., 2011). Mint (Mentha piperita L.) oil also showed antifungal activity against Aspergillus niger, Alternaria alternata and Fusarium chlamydosporum (Lirio et al., 1998; Aqil et al., 2001).Yeasts are found within the surface microflora of many cheese types. Within 2 or 3 days of ripening, growth of salt-tolerant yeasts appears on the cheese surface. However, occasionally fungal growth discolours the cheese surface and affects the flavour. During fungal growth a risk of mycotoxin production in the cheese exists. For example Northolt et al. (1980) investigated cheeses infected by Aspergillus versicolor and contained sterigmatocystin.Taxonomic and phylogenetic overviews of the most important filamentous cheese fungi were presented by Roparset al. (2012). Some fungi, such as, P. camemberti, F. domesticum, Scopulariopsis flava and S. casei, are only known from cheeses and are probably adapted to this particular habitat, which is extremely rich in protein and fat. Other cheese fungi are ubiquitous, such as, P. roqueforti, Scopulariopsis candida and Scopulariopsis fusca (Roparset al. 2012).The present study aimed at evaluating the effect of three essential oils from Laurus nobilis, Anethum graveolens and Mentha piperita on the organoleptic characteristics and mycobiota that might be associated with low salt white soft cheese during storage. Chemical analysis of these oils to evaluate their major components was also targeted.
2. Materials and Methods
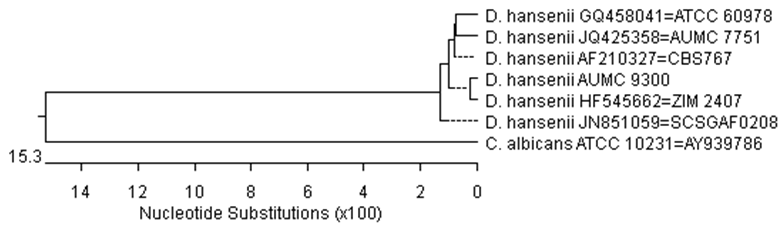
- Source of Laurus nobilis, Anethum graveolens and Mentha piperita oilsThe oil extracts of Laurel (Laurus nobili L., Family Lauraceae) L. nobilis, Dill (Anethum graveolens L., Family Apiaceae) and Mint (Mentha piperita L., Family Lauraceae) were purchased from plants medical group, Cairo, Egypt.Cheese makingFresh raw buffaloe’s milk having 13.4% total solids, 4.90% fat, 3.60% protein, and 0.18% titratable acidity was obtained from private farm in Assiut Governorate during the month of November 2012. Commercial liquid calf rennet and commercial salt were obtained from local markets. Domiati cheese was made as described by Fahmi and Sharara (1950). Milk retentate was divided into 10 portions; each portion was salted to a concentration of 3%, well mixed and pasteurized at 73°C for 15 sec. First portion was served as control and for the other nine portions, different concentrations of L. nobilis, A. graveolens and M. piperita essential oils (3, 5, and 7 ml / 100g retentate) were added at 40°C to prepare cheese treatments. Curds were hold at the same temperature after adding the rennet, cheeses samples were taken for analysis at intervals during storage in refrigerator (5±1°C) for up to 11 weeks. Three replicates were prepared for each cheese sample to determine their mycological analysis and organoleptic properties were also assessed.Chemical analysis of oils using Gas chromatography- mass spectrometry (GC-MS)The essential oils were analyzed using Gas Chromatography/Mass Spectrometry (GC/MS). A Hewlett-Packard (HP) system 6890 series gas chromatograph coupled with a HP model 5975B quadruples mass spectrometer; cross-linked 5% phenyl methyl siloxane capillary column (HP-5MS, 30 m x 0.25 mm x 0.25 µm film thickness) was used. GC operating conditions were as follows: initial temperature 40ºC (1 min hold), increased at 20ºC min-1 to 210ºC, then increased at 1.5ºC min-1 to 215ºC (4 min hold); injector temperature 240ºC; carrier gas Helium (99.999%), flow-rate 1.3 ml-1 min; ion source temperature 270ºC; operated in the split less mode; purge off time 1 min; injection volume 1 µl. MS operating conditions were: solvent delay 6 min; electron-impact (EI) mode ionization voltage 70 eV using selected ion monitoring (SIM); dwell time for each ion 100 ms (EPA 1998).Sensory analysisFifteen panelists (7 males and 8 females, aged between 25 and 45 years) who have experience with white cheese and regularly used its descriptive vocabulary, were participated. The cheese samples were scored for flavor (50 points), body and texture (40 points) and appearance and colour (10 points) (Larmond 1977). Panel members were also instructed to report any defects or unpleasant flavor.Fungal assessmentThree concentrations (3, 5, and 7 ml/ 100g retentate) of each of three natural oils were added at the beginning of manufacturing of low salt white cheese. A sample of cheese without natural oils was used as a control. Cheese samples were analysed firstly at the beginning of manufacturing (8 hours after adding oil), then after 3, 7 and 11 weeks (until cheese got spoiled). Dilution-plate method was used for isolation of fungi. Dichloran rose-bengal chloramphenicol agar (DRBC) was used as a general medium for isolation of fungi (King et al. 1979). 1 ml of an appropriate dilution of an in vitro manufactured low-salt white cheese sample was transferred into sterilized petri dish, then 15-20 ml sterilized molten DRBC medium were poured. The plates were incubated at 28ºC for 7-10 days. The fungal colonies were identified based on morphological and microscopic characters according to Pitt and Hocking (2009). Microscopic examination of preparations of the different fungal species stained with lactophenol cotton blue were carried out. Yeasts were identified by molecular methods. The ITS sequences of nuclear rDNA were amplified using ITS1 ((5' - TCC GTA GGT GAA CCT GCG G - 3'),, and ITS4 ((5'- TCC TCC GCT TAT TGA TAT GC -3') primers in SolGent company (Daejeon, Stouth Korea). Contigs were created from the sequence dat using CLC Bio Main Workbench program. The sequence obtained from the isolate was further analyzed using BLAST from the National Center of Biotechnology Information (NCBI) website. Sequences obtained were subjected to Clustal W analysis using MegAlign (DNAStar) software version 5.05 for the phylogenetic analysis and the phylogenitic tree was constructed.Screening for aflatoxinsSeven isolates of Aspergillus flavus (3) and some other fungal species (4) collected in this study were screened for aflatoxin production using coconut agar medium (CAM). CAM was prepared according to Davies et al. (1987) as follows; 100 g of shredded coconut was homogenized for 5 min with 300 ml hot distilled water, then the homogenate was filtered through four layers of cheese cloth, completed into 1000 ml by distilled water, the pH of the clear filtrate was adjusted to pH 7 and 20 g agar were added. The medium was autoclaved for 15 min at 121ºC, cooled to about 40-45ºC, and poured while being stirred into sterile Petri-dishes. Fungal isolates were inoculated at the center of CAM agar plates and incubated at 25ºC in the dark for 7 days. Cultures were observed for blue fluorescence under long-wave (365 nm) UV light. An uninoculated plate was observed as a reference.Statistical analysis Statistical analysis of experimental data was performed by analysis of variance (ANOVA) producers using SPSS version 9.0 program to examine statistical significance differences of sensory analysis means of experimental data. Results were considered statistically significant when p < 0.05. Mean ± standard deviation values were also presented.
3. Results and Discussion
- GC-MS analysis of oilsUsing the essential oil (EO) as a natural preservative have been increased the researches provided that the biologically active compounds in EO have been seen as a potential way to control fungal contamination. The present study had assessed potential antifungal activity of essential oils Laurus nobilis, Anethum graveolens and Mentha piperita. GC-MC analysis of L. nobilis oil resulted in 21 major components with concentrations ranging from 10.7% (for γ-cadinene) to 0.5% (for elemenal). These components and their concentrations as revealed by GC-MS analysis in the current study along with their antifungal effects (Kurtulmus et al., 2009 & Rodrigues et al., 2012 & Policegoudra et al., 2012 & Costa et al., 2013).Also, GC-MS analysis of A. graveolens oil resulted in 13 major components with concentrations ranging from 31.9% (for parsley camphor) to 0.7% (for α- patchoulene). These components and their concentrations as revealed by GC-MS analysis are presented in table (2). The antifungal effects reported earlier by these components were also presented in table (2).
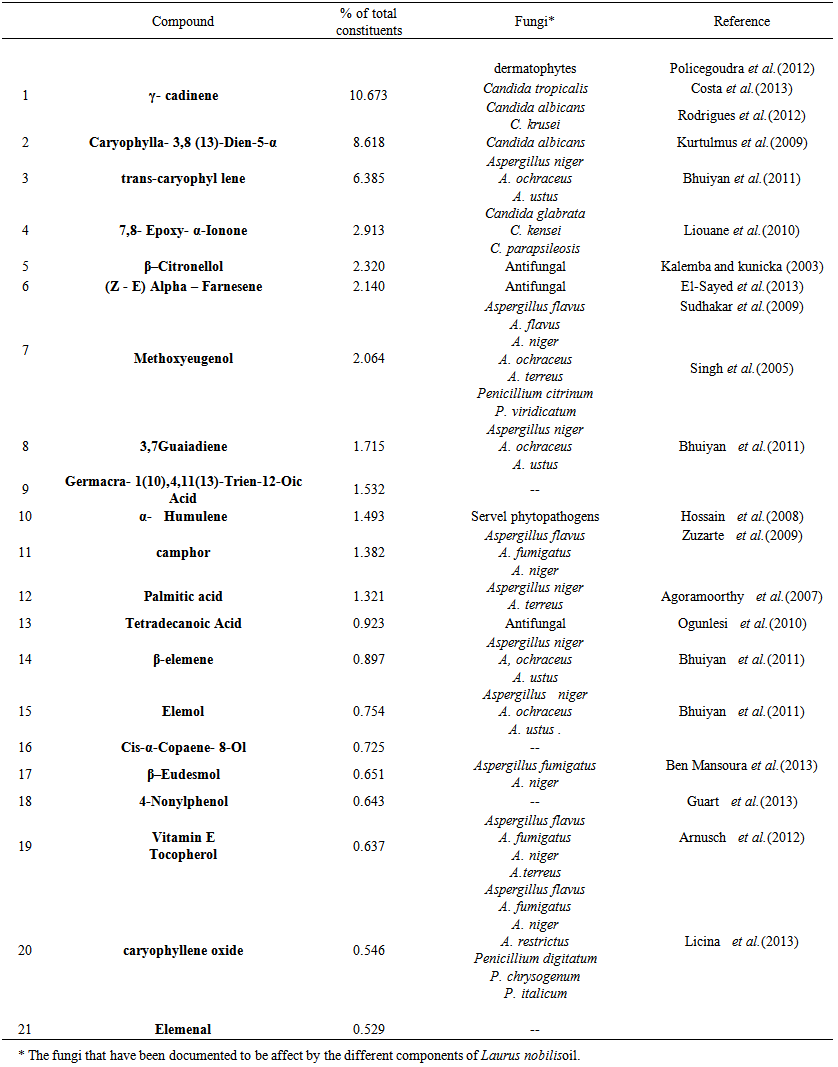 | Table 1. The major components and their percentages of Laurus nobilis oil, as revealed byGC- MS analysis |
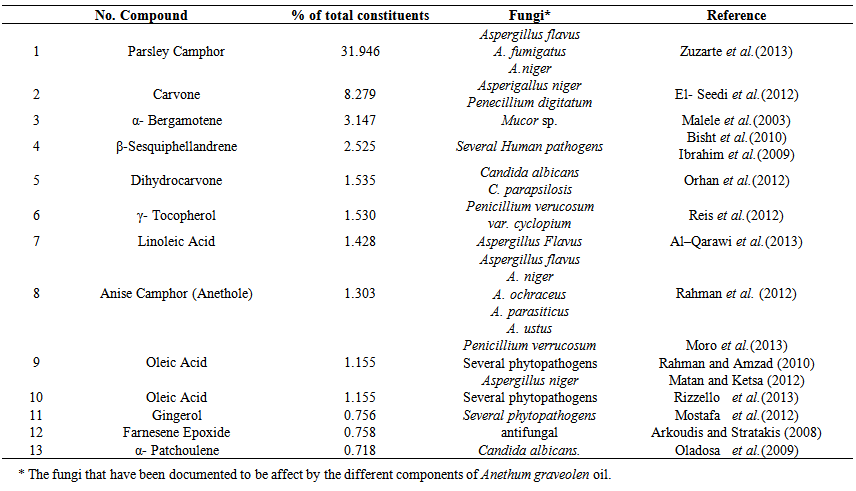 | Table 2. The major components and their percentages of Anethum graveolen oil, as revealed byGC- MS analysis |
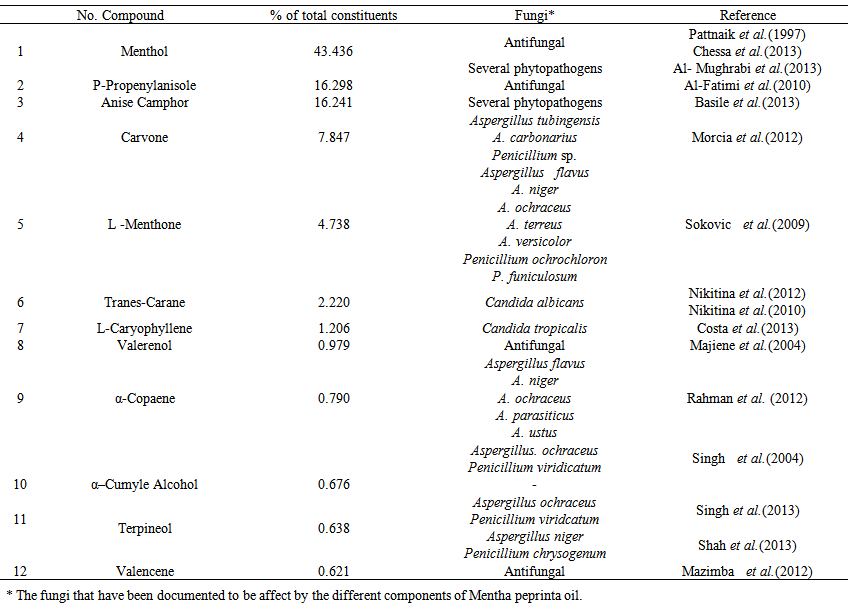 | Table 3. The major components and their percentages of Mentha peprinta oil, as revealed by GC- MS analysis |
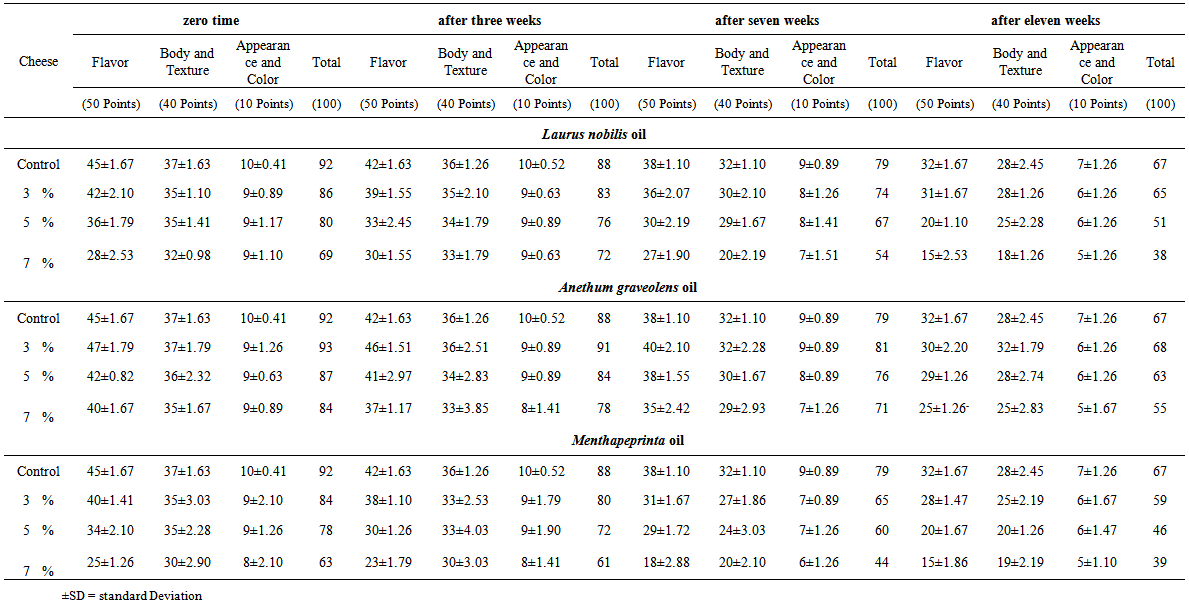 | Table 4. Organoleptic characteristics of domiati cheese furcated with different concentration of Laurus nobilis, Anethum graveolens and Mentha piperita oils |
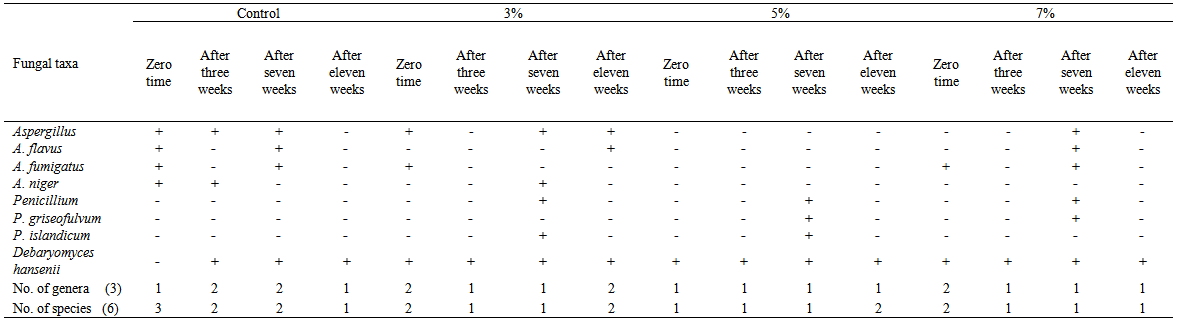 | Table 5. Fungi isolated on dichloran rose-Bengal agar (DRBC), from domiati cheese samples, supplemented with three concentrations (3, 5, 7%) of Laurus nobilis oil |
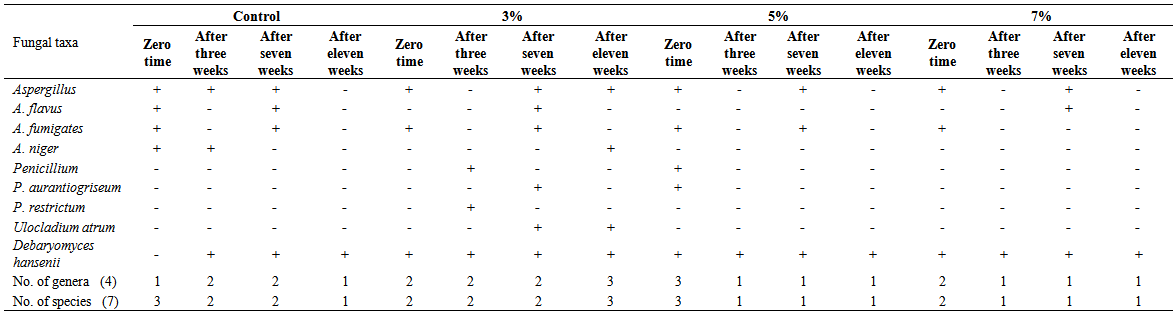 | Table 6. Fungi isolated on Dichloran Rose-Bengal Agar (DRBC), from domiati cheese samples, supplemented with three concentrations (3, 5, 7%) of Anethum graveolens oil |
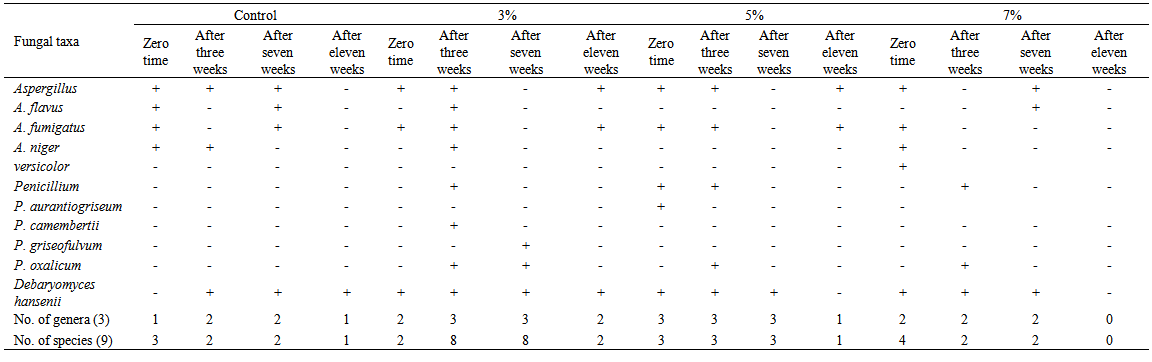 | Table 7. Fungi isolated on Dichloran Rose-Bengal Agar (DRBA), from domiati cheese samples, supplemented with three concentrations (3, 5, 7%) of Mentha piperita oil |
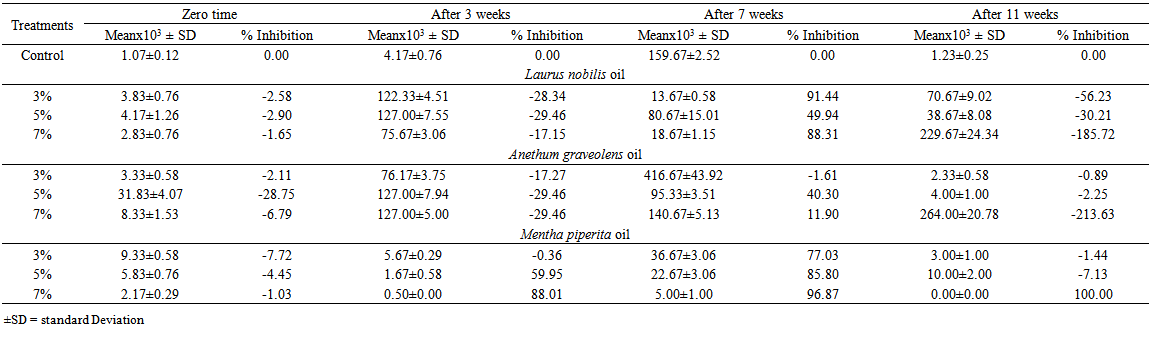 | Table 8. Mean, standard deviation (SD) and percent of inhibition in total counts of fungi isolated from Domiati cheese on DRBC medium |
4. Conclusions
- Generally, results showed that treatment of cheese with M. piperita oil significantly decreased total counts of fungi. On the other hand, oils of L. nobilisand A. graveolens caused either increase or decrease in fungal counts. Twelve species appertaining to 4 genera of fungi were isolated from cheese and cheese treated with essential oils. The results revealed that the Domiati cheese under investigation exhibited low level of contamination and most of the samples tested, were free of fungi, where the total fungal counts ranged from 95 and 125 colonies/160 cheese pieces.
ACKNOWLEDGEMENTS
- This research was financially supported by Assuit University, Egypt.
Note
- 1. Web: http://www.aun.edu.eg/membercv.php?M_ID=1261
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML