-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2014; 4(3): 87-92
doi:10.5923/j.fph.20140403.03
Physicochemical Characterization and Thermal Properties of Lipids from R. opacus PD630
Shuo Yang 1, Robin S. Simmonds 2, Edward J. Birch 1
1Department of Food Science, University of Otago, PO Box 56, Dunedin, 9054, New Zealand
2Department of Microbiology and Immunology, University of Otago, PO Box 56, Dunedin, 9054, New Zealand
Correspondence to: Edward J. Birch , Department of Food Science, University of Otago, PO Box 56, Dunedin, 9054, New Zealand.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Physicochemical properties of lipid from R. opacus PD630 were studied along with positional distribution of triacylglycerol fatty acids and thermal properties of the oil. Results showed that saturated fatty acids (SFA) contribute a large portion (51.50 ± 0.49%) of total fatty acids. A high proportion of fatty acids with an odd number of carbon atoms (32.08 ± 0.39%) were also detected. The lipid was high in red and yellow colour parameters and contained a high level of unsaponifiable matter. It was oxidatively stable and had good quality based on its moisture and volatile content, peroxide value, p-anisidine value, TOTOX value, conjugated diene and triene values, free fatty acid and acid values. The SFA (70.56 ± 1.95%) were enriched at sn-2 position against sn-1,3 position while unsaturated fatty acids were predominantly found at sn-1,3 position. The melting and crystallization of lipid from R. opacus PD630occurredover a large temperature range from -7.45°C to 32.37°C and 17.62°C to -19.93°C respectively, due to its high degree of saturation. Lipids from R. opacus PD630 decomposed in four stages during programmed heating, comprising decomposition of unsaponifiable matter, monounsaturated fatty acids (MUFA) and SFA degradation, followed by decomposition of MUFA and SFA degradation products. Based on the data, lipid from R. opacus PD630 can be considered as a good quality oil and is thermally stable.
Keywords: Physicochemical properties, Thermal properties, Oil quality, R. opacus PD630
Cite this paper: Shuo Yang , Robin S. Simmonds , Edward J. Birch , Physicochemical Characterization and Thermal Properties of Lipids from R. opacus PD630, Food and Public Health, Vol. 4 No. 3, 2014, pp. 87-92. doi: 10.5923/j.fph.20140403.03.
Article Outline
1. Introduction
- Microbial oils have been receiving increasing attention as a source of novel oils. Microorganisms accumulating more than 20-25% of their biomass as oil may be termed as oleaginous and their oils as single cell oils, unicellular oils or microbial oils. Microbial oils have a number of potential commercial applications as nutraceuticals, pharmaceuticals, feed ingredients for aquaculture as well as feedstock for producing biodiesel [1]. The major hindrance to their commercialisation is their high cost of production. Rhodococcusopacus strain PD630 is an oleaginous microorganism species, which has the ability to synthesize a large percentage of oil. In R. opacus PD630, the triacylglycerols (TAG) are synthesized in the cytoplasm as insoluble inclusions from several carbon sources such as carbohydrates, alkanes or fatty acids. They can accumulate up to 76% of the cellular dry matter, probably the highest TAG content ever found in bacteria. The lipid from R. opacus PD630 was reported to contain an unusual fatty acid composition. The TAG was composed of fatty acids with 14-18 carbon atoms with a large portion of odd numbered fatty acids [2, 3]. Recent studies have shown the anticarcinogenic effects of odd- and branched-chain fatty acids on cancer cells and antifungal effect of odd numbered fatty acids [4, 5].Information on thermal properties is important in lipid analysis to provide information about functionality and application of the lipids [6]. Differential Scanning Calorimetry (DSC) is a thermal technique to measure heat flow and heat capacity of lipid samples during phase transitions, including melting and crystallization, which are associated with chemical composition [6]. Thermal decomposition can be monitored by thermogravimetric analysis (TGA) through weight change under heating. A higher temperature of decomposition suggests a higher thermal stability [7].The biotechnological relevance of TAG is due to the production of novel lipids that are different to those already available from animals and crops [8]. R. opacus PD630 can become an interesting candidate for the biotechnological production of “high-value single-cell lipid” as well as other novel lipids such as branched-chain fatty acids that can be utilized for food technology and other specialized applications if genetically modified strains of this species are applied [9]. The aim of this study was to characterize the physicochemical properties of lipid from R. opacus PD630, to determine positional distribution of fatty acids in the lipid and to study its thermal properties.
2. Materials and Methods
2.1. Materials
- R. opacus PD630 (DSM 44193) cells were prepared in the Department of Microbiology and Immunology, University of Otago, New Zealand. Solvents and reagents were all of analytical grade.
2.2. Methods
2.2.1. R. opacus PD630 Cultivation and Harvest
- R. opacus PD630 cultivation and harvest was conducted according to a modified [10] method. The culture media used were brain heart infusion (BHI) broth and a phosphate-buffered defined medium containing 400 g sucrose, 21 g (NH4)2SO4, 10 g MgSO4.7H2O, 113.3 mg CaCl2, 5 ml antifoam, 0.35 mol phosphate buffer, 10 ml trace element solution, and 10 ml stock A solution per liter. Phosphate buffer, stock a solution and trace element solution were the same as those described [11].BHI broth was inoculated with R. opacus PD630 and incubated at 30°C on a shaking incubator at 200 rpm for 72 hours. Cells were harvested by centrifugation at 4000 rpm for 15 minutes, resuspended in sterile saline, and added into sterilized phosphate-buffered defined medium. The mixture was added into the fermentor (BioFlo 410; New Brunswick Scientific Inc., USA) and cultivated at 30°C with ambient air supply at 10 L/m (1 vvm), pH 7.0 control with 2 M NaOH and shaking (200 rpm) for 72 hours. At the end of the cultivation, biomass was collected by centrifugation at 4000 rpm for 15 minutes. The retained wet cell mass was resuspended in 500 ml of reverse osmosis water.
2.2.2. Lipid Extraction
- The crude lipids were extracted by mixing chloroform-methanol (1:1 v/v) with the resuspended R. opacus PD630 cells in the proportion 2:1 according to a modified method [12]. The mixtures were centrifuged at 3000 rpm for 10 minutes and the chloroform layer was collected and evaporated by rotary vacuum evaporator to obtain the crude lipid.
2.2.3. Fatty Acid Composition
- Fatty acids were analyzed as fatty acid methyl esters (FAME) according to a modified [13] method. Lipid sample (50 mg) and internal standard (C10:0; 1 mg) was mixed with KOH in methanol (0.5N; 2 ml) and saponified at 80°C for 2.5 hours. Diethyl ether (2 ml) and 5 ml of milli-Q water were added into the mixture. The aqueous phase was acidified with hydrochloric acid (37%) until it was red to litmus paper. The diethyl ether phase (upper phase) was collected and added into 2ml of boron trifloride (14%) in methanol and heated at 80°C for 20 minutes. Saturated NaCl (5ml) solution was added into the mixture. The upper phase containing FAME was collected for gas chromatography (GC) analysis. A BP70 capillary column (50 m × 330μm × 0.25μm) was used for separation of the FAME using gas chromatography-flame ionization detection (Agilent 6890N; Agilent Technologies Inc., USA) with a split ratio of 20:1. Hydrogen was used as the carrier gas and the flow rate was 2.2 ml/min. The temperatures of injector and detector were 250°C.The temperature settings were as follows: 70°C to 230°C at 5°C/min and isothermal at 230°C for 20 minutes. The fatty acids were identified using a FAME reference standard (FAMQ-005). HP Chemstation computer software was used for data analysis.
2.2.4. Triacylglycerol Positional Distribution of Fatty Acids
- Analysis of positional distribution of fatty acids was conducted according to a modified method [14]. Lipid sample (100mg) was mixed with 3 ml of buffer containing 1M of tris (hydroxymethyl)-amino methane pH8.0, 0.15M CaCl2, and 0.01% bile salts in a test tube. Pancreatic lipase, Type II (200 mg) was mixed with 5ml tris buffer, and 0.5ml of this mixture was added into the test tube. The test tube was incubated at 37°C on a shaking incubator at 250 rpm for eight minutes. Diethyl ether (3 ml) was added for phase separation. The diethyl ether phase was transferred into a new test tube, back-washed with 2 ml of milli-Q water and concentrated under a stream of nitrogen gas.The concentrated diethyl ether and the neutral lipid standards were spotted on a glass silica gel thin layer chromatography (TLC) plate (silica gel 60 F254) which was previously washed with hexane-diethyl ether (50:50, v/v). Separation of lipid classes was conducted using a hexane-diethyl ether-acetic acid (50: 50: 1, v/v/v) solution. The plate was left to dry at room temperature when the solvent had moved up to 90% to the top of the plate. The TLC plate was sprayed with 5% phosphomolybdic acid in ethanol and placed under UV light to observe the lipid fractions. The monoacylglycerol and free fatty acid bands were marked and scraped off the plate for the methylation process and analysis by GC as outlined in section 2.2.3.
2.2.5. Colour
- Analysis of oil colour was carried out using a MiniScan XE spectrocolourimeter (HunterLab) with a measuring head hole of 22mm, D65colour illuminant and 10° observer. Colour intensities were measured using the CIELAB (L*, a*, b*) colour scale.
2.2.6. Determination of Physical and Chemical Properties
- Moisture and volatile matter content, unsaponifiable matter content, peroxide value, p-anisidine value, free fatty acid content, and acid value were determined at 25°C according to [15] methods. Specific extinctions of lipids were determined spectrophotometrically at 230 and 270 nmas outlined [16].
2.2.7. Thermal Analysis
- Melting and crystallization characteristics of lipid from R. opacus PD630 were measured by DSC (Q2000, TA instruments Ltd., USA) using sealed aluminum pans (Tzero pan and lid) with a sample of 10mg. The experiments were carried out over the temperature range -50°C to 60°C at 2°C /min under nitrogen atmosphere (50 ml/min). The DSC instrument was calibrated using indium (melting point = 156.6°C, enthalpy = 28.45 J/g) according to the DSC instrument manual. Melting and crystallization profiles, transition enthalpies and solid fat content (SFC) of the lipid sample were produced using TA Universal Analysis 2000 software.The thermal decomposition process was monitored by TGA (Q50, TA instruments Ltd., USA). Samples (10 to 15mg) were heated from 10°C to 700°C at 2°C /min in an air atmosphere. Data on the percentage of weight change were obtained using the TA Universal Analysis 2000 software. The onset temperature for decomposition was extrapolated from the thermal decomposition curve according to [7].
2.2.8. Statistical Analysis
- All measurements were carried out in triplicate. Data were reported as mean ± standard deviation (SD). Statistical analysis of data was performed using IBM SPSS Statistics Version 20. One way ANOVA and Tukey’s test were used to determine significant difference between samples for each measurement at p<0.05.
3. Results and Discussion
3.1. Fatty Acid Composition
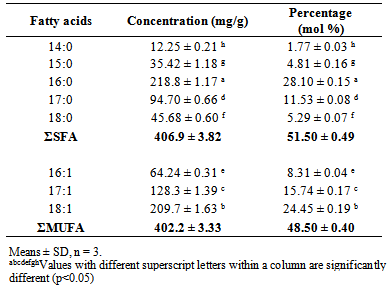
- The fatty acid composition of lipid from R. opacusPD630 is shown in Table 1.The most dominant fatty acid was palmitic acid (C16:0), which contributed 28.10 ± 0.15% of total fatty acids followed by oleic acid (C18:1). The result confirms the relatively large proportion of margaroleic acid (C17:1; 15.74 ± 0.17%) and palmitoleic acid (C16:1; 8.31 ± 0.04%) which may give health benefits. SFA contributed 51.50 ± 0.49% of total fatty acids, while monounsaturated fatty acid (MUFA) contributed 48.50 ± 0.40%. There were no polyunsaturated fatty acids found in lipid from R. opacusPD630. A large portion (32.08 ± 0.41%) of odd carbon numbered fatty acids was found in the lipid. The data agrees with the results from previous studies conducted [2, 3, 17].
|
3.2. Positional Distribution
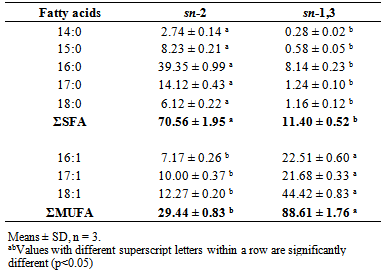
- The results of positional distribution of the fatty acids are shown in Table 2. The results show the saturated fatty acids (SFA) prefer sn-2 position against sn-1,3 position while unsaturated fatty acids were predominantly found at sn-1,3 position. The results of the present study were similar to previous results [3]. However, there were more MUFA found at sn-2 position, but less SFA found at sn-1,3 position in the present study due to the different carbon source used for R. opacus PD630 cultivation. The positional distribution of the fatty acids in lipids of R. opacusPD630 are similar to animal fats as SFA are predominantly found at sn-2 position in most animal fats, whereas MUFA prefer sn-2 position against sn-1,3 position in vegetable oils [18].
|
3.3. Colour
- Table 3 shows the CIELAB L*a*b*colour values of lipid from R. opacusPD630. The a* and b* values of lipid from R. opacusPD630 (15.37, 19.14) were higher than that of vegetable oils including olive oil (68.3, 3.1), sunflower oil (65.8, 3.4), corn oil (66.4, 4.1) and palm oil (64.5, 4.0), which suggest that the lipid contained a higher level of yellow pigments [19]. The L* value of lipid from R. opacus PD630 (49.33) was lower than that of olive oil (9.9), sunflower oil (10.0), corn oil (10.0) and palm oil (9.6), which suggests that lipid from R. opacus PD630 was darker compared with vegetable oils [19].
|
3.4. Quality Characteristics of Lipid from R. opacus PD630
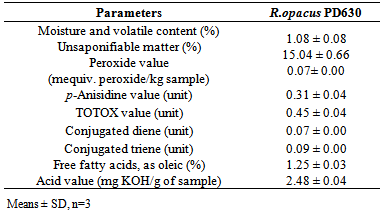
- Table 4 shows the physicochemical characteristics of lipid from R. opacus PD630. The percentage of moisture and volatile matter (1.08 ± 0.08%) was higher compared to that of vegetable oils (0.60-0.72%) including hemp seed oil, canola oil and flaxseed oil [20]. A large amount (15.04 ± 0.66%) of unsaponifiable matter content was found in the oil sample. It is desirable for good quality oil to have low moisture and unsaponifiable matter content [20].
|
3.5. Thermal Analysis
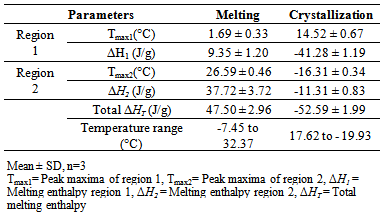
- Figure 1 shows the melting profile of lipid from R. opacus PD630. There were two endothermic regions of the melting curve. The first endothermic region at 1.69 ± 0.33°C (Table 5) represents the melting of MUFA and the second endothermic region at 26.59 ± 0.46°C indicates the contribution of SFA. The peak shapes are the result of overlapping effects from composition and polymorphism [26]. The total melting enthalpy for lipid from R. opacus PD630 was 47.50 ± 2.96J/g. The melting enthalpy of the second region (37.72 ± 3.72 J/g) was higher than that of the first region (9.35 ± 1.20 J/g). This is due to unsaturated fatty acids not aligning as neatly as SFA due to their cis double bonds, hence requiring less energy to overcome the intermolecular attraction, and melting first.
|
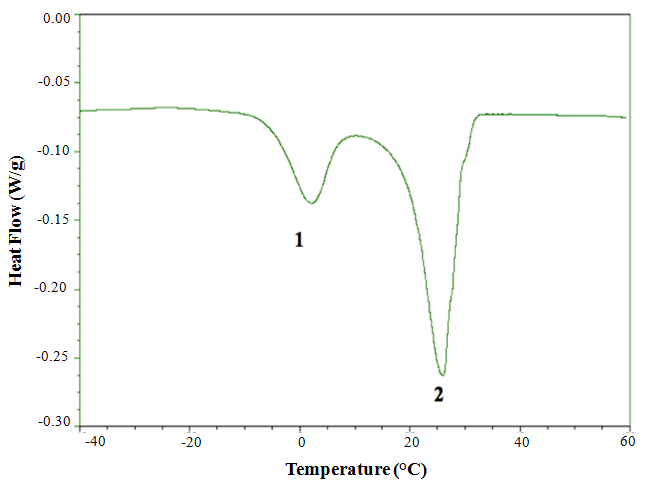 | Figure 1. DSC melting profile of lipid from R. opacus PD630 |
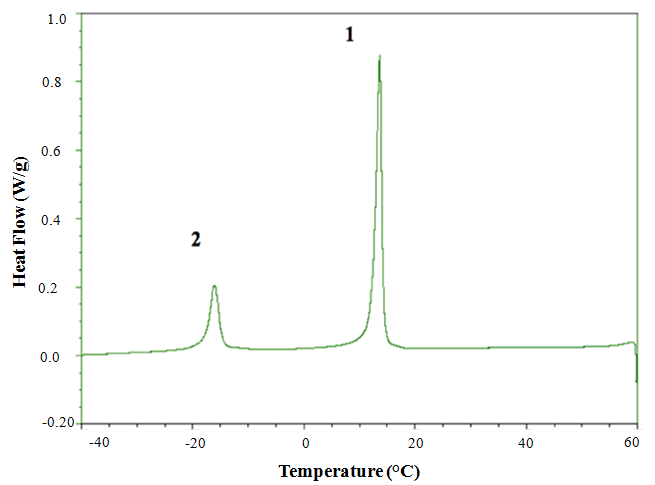 | Figure 2. DSC crystallization profile of lipid from R. opacus PD630 |
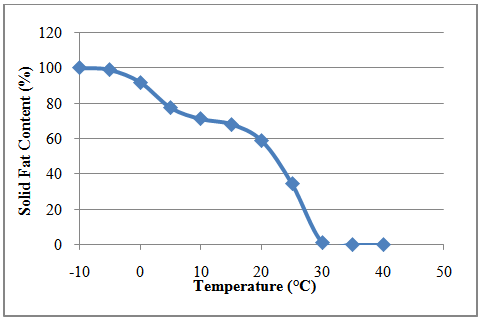 | Figure 3. Solid fat content of lipid from R. opacus PD630 |
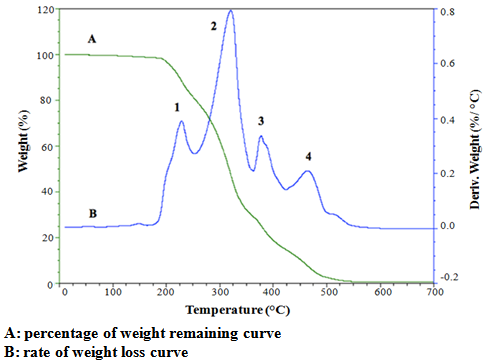 | Figure 4. Thermal decomposition of lipid from R. opacus PD630 by TGA from 10°C to 700°C |
4. Conclusions
- In conclusion, lipid of R. opacusPD630 in the present study was less susceptible to lipid oxidation than vegetable oils in previous studies due to the high SFA content, as reflected by its low peroxide value, p-anisidine, TOTOX value, free fatty acid content, acid value, conjugated diene and triene value. However the unsaponifiable matter content of lipid is much higher than most animal and vegetable lipids. The colour parameters suggest that the lipid from R. opacus PD630 was darker, redder and more yellow compared with vegetable oils. In TAG of R. opacusPD630, SFA were predominantly found at sn-2 position and unsaturated fatty acids tend to prefer the sn-1,3 position against the sn-2 position, which is similar to animal fat. The lipid of R. opacus PD630 showed a wide melting and crystallization range. Thermal decomposition of lipid from R. opacus PD630 exhibited four stages that represent the decomposition of unsaponifiable matter content, MUFA, SFA and their degradation products. Based on the above data, lipid from R. opacus PD630 can be considered as good quality oil which is also thermally stable. The unusually high content of margaric and margaroleic acid warrants further investigation for potential health benefits.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML