-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Food and Public Health
p-ISSN: 2162-9412 e-ISSN: 2162-8440
2014; 4(3): 82-86
doi:10.5923/j.fph.20140403.02
Sanitary and Bacteriological Studies of Different Aquatic Environments in Ibadan, Nigeria
Tiamiyu Adebisi Musefiu , Soladoye Mike Olasunkanmi , Adegboyega Taofeek Tope , Adetona Mutiat Olutope
Department of Biological Sciences, Southwestern University Nigeria, P.O BOX 2088, Okun-Owa, Ijebu Ode, Ogun State, Nigeria
Correspondence to: Tiamiyu Adebisi Musefiu , Department of Biological Sciences, Southwestern University Nigeria, P.O BOX 2088, Okun-Owa, Ijebu Ode, Ogun State, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
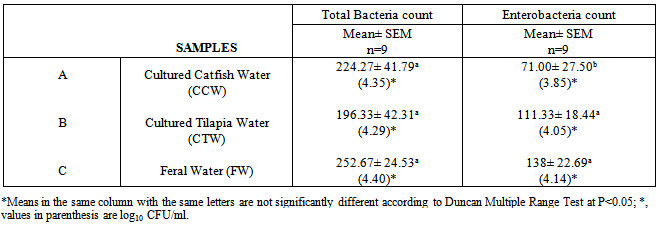
The physico-chemical properties and microbial quality of water used for the culturing of fishes play important role in management of aquacultures. The physico-chemical attributes and bacteriological quality of different aquatic environments in Ibadan, Southwest, Nigeria were studied. The result revealed higher significant difference of total dissolved solid (>68.3%, p<0.05) in feral water. Lower significant difference of dissolved oxygen saturation (<0.15mg/L, p<0.05) was obtained in pond water used for culturing Nile tilapia, while ammonia was not detected in any of the aquatic environments studied. Others parameters were indifferent but could support bacteria proliferation particularly temperature ranged from 30.5-31.5℃. The heterotrophic bacteria count obtained from different aquatic environments ranged from 196.33-252.67x102 (log 10 cfu/cm2 4.29 -4.40). The highest microbial load of 252.67x102 cfu/ml was recorded in the feral water and this could be due to heavy environmental pollution. Staphyloccous, Esherichia coli, Pseudomonas sp, Klebsiella sp, Streptococcus sp, Salmonella sp and Proteus sp were all isolated from the water samples from different aquatic environments. The bacterial assemblage was of public health significance.
Keywords: Aquatic environments, Bacteria, Physico-chemical parameters, Public health
Cite this paper: Tiamiyu Adebisi Musefiu , Soladoye Mike Olasunkanmi , Adegboyega Taofeek Tope , Adetona Mutiat Olutope , Sanitary and Bacteriological Studies of Different Aquatic Environments in Ibadan, Nigeria, Food and Public Health, Vol. 4 No. 3, 2014, pp. 82-86. doi: 10.5923/j.fph.20140403.02.
Article Outline
1. Introduction
- As the human populations continue to expand, its reliance on captured and farmed fish production as important source of protein will also increase. Fish like other aquatic animals largely depend on aquatic environments. Water quality is the main factor that determines the degree of production and quality of the fish products. The physico-chemical and biological properties of water play a significant role in the sanitary and bacteriological quality of water (Dorota, et al., 2002). In Nigeria, the deposition of human, animal excreta and other environmental wastes into natural water and run-off containing fecal matter of various sources during rainy seasons ultimately emptied into water bodies are capable of elevating bacterial counts in water bodies and ponds (Doyle and Erickson, 2006). It has been known that the microorganisms associated with most fishery products indicate the microbial flora in their aquatic environments (Ashie et al., 1996; Gram and Huss, 1996). Fishes which are farmed in poor and or polluted water are not only prone to diseases but can be of health hazard to handlers and consumers (Boyd, 1998). In previous studies on Lagos lagoon (Ekundayo , 1997), on Ele river (Ajiwe et al., 2000) and on Otamiri river (Ibe and Ozor 2000), different bacterial species with potential for causing high proportion of death and ill- health in population dependents on the water bodies for water related resources were identified. While majority of published works in Nigeria are on fish with few or none on physico-chemical properties and bacteriological quality of aquatic environments, the authors of this current research undertook this study to produce data on physcio-chemical properties and bacteria occurrence to determine the contamination level and sanitary state of different aquatic environments in Ibadan South west Nigeria.
2. Materials and Methods
2.1. Study Location
- Three study sites in Ibadan, South west, Nigeria were used for this study. A commercial farm fish ponds culturing Clarias gariepinus (African catfish) and a fishery institute ponds culturing Oreochromis niloticus) (Nile Tilapia), both situated in Ibadan South west Local Government Area, Oluyole Estate, Ibadan, and Eleyele river, a major source of different aquatic animals in Ibadan North West Local Government Area, Onireke Ibadan, Nigeria.
2.2. In Situ Measurement of Physico-chemical Parameters of Water Samples
- At the site of the river, and cultured ponds, dissolved oxygen (DO), water temperature (℃), ammonia (NH3), pH and total dissolved solids (TDS) were measured using a portable Hach meter (Hach Company, Loveland, USA), and Aquachek® (USA) water quality test strips. Mean readings were taken for Morning (8.00 a.m) and afternoon (4.00 p.m) for three (3) consecutive times at periodic interval of seven days. The probe of HACH meter was dipped inside the tested water, and stabilized readings of DO, pH, T℃, and Total Dissolved (TDS) displayed on the meter monitor immediately were recorded. Aquacheck® stripes were manually dipped inside the tested water. For pH reading, the stripe was removed immediately; for Ammonia, the stripe was vigorously moved up and down inside the tested water for 30 seconds. Colour change for pH and NH3 were read against the standards colours in 15 seconds and 30 seconds respectively.
2.3. Collection of Water Sample
- Water samples were drawn in sterile 500ml bottles from the three different sites of each pond and river in each occasion of sampling. The water samples were brought to the laboratory in ice box at temperature below 4℃ within 2 hrs of sampling. Samples were drawn between 8.00 and 10.00am for morning and 3.00-4.00pm for afternoon.
2.4. Processing of Water Samples
- Each water sample collected from river and cultured ponds was analyzed and total bacterial count (TBC) and Enterobacteriaceae count (EC) were determined. In brief, for the enumeration of TBC and EC in water, 1ml sample water was mixed with 9 ml of sterile normal saline (0.85% NaCl solution) to prepare 10-1 dilution. Then 0.1ml of diluted water samples was inoculated on Nutrient Agar (NA) and MacConkey Agar (MAC) for enumeration of TBC and EC respectively. The plates were then incubated at 37℃ for 18-24 hrs. The distinct colonies were counted. The distinct colonies were further sub-cultured on freshly prepared NA and MAC for colonial purification. The isolates were equally identified using Gram-staining method, physiological, biochemical reactions and fermentation of sugar according to standard taxonomic schemes (Buchanan and Gibbons, 1974).
2.5. Statistical Analysis
- The bacterial density data were transformed into natural log before statistical analysis. The means of physico-chemical parameters and bacterial load were compared by using analysis of variance (ANOVA) and significant means separated using Duncan multiple range test (DMRT) as outlined by Steels and Torrie (1980). The values of p<0.05 were considered significant.
3. Results and Discussion
- The physico-chemical attributes of water samples of different aquatic environments are as presented in Table 1. The temperature of the water ranged from 30.5-31.5℃ with higher values obtained in the noon. The pH values in the range of 7.33-7.50 were obtained in study. The pH is interdependent with other water quality parameters such as carbon dioxide, alkalinity and hardness. pH can be toxic in itself at a certain level and also known to influence the toxicity as well of hydrogen sulphide, cyanides, heavy metals and ammonia (Klont,1993). A pH between 6.5-9.0 is ideal for freshwater animal (Boyd, 1998). Below pH 6.5, some species expresses slow growth and some organism’s ability to maintain salts balance is affected (Lloyd, 1992).
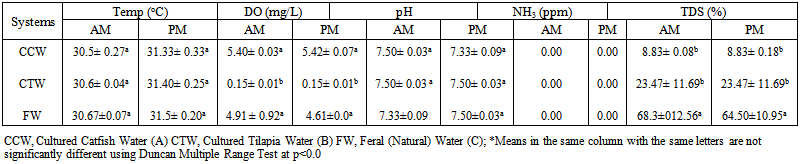 | Table 1. Physico-chemical properties of the different aquatic environments |
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML